Get the free Transfusion Reaction Investigation Form (CM105) - gov mb
Show details
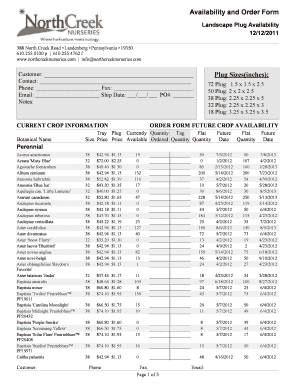
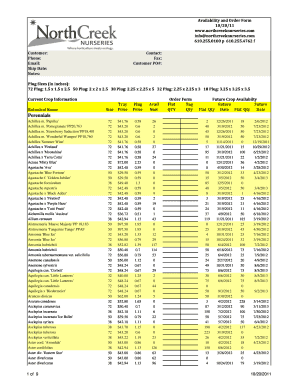
This document provides examples of ISBT 128 labels for various blood products, outlining the necessary information to record on the Transfusion Reaction Investigation Form (CM105).
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign transfusion reaction investigation form

Edit your transfusion reaction investigation form form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your transfusion reaction investigation form form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing transfusion reaction investigation form online
Follow the guidelines below to benefit from the PDF editor's expertise:
1
Create an account. Begin by choosing Start Free Trial and, if you are a new user, establish a profile.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit transfusion reaction investigation form. Rearrange and rotate pages, insert new and alter existing texts, add new objects, and take advantage of other helpful tools. Click Done to apply changes and return to your Dashboard. Go to the Documents tab to access merging, splitting, locking, or unlocking functions.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
With pdfFiller, it's always easy to deal with documents. Try it right now
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out transfusion reaction investigation form

How to fill out Transfusion Reaction Investigation Form (CM105)
01
Begin by entering the patient's identification details at the top of the form.
02
Fill in the date and time the transfusion reaction occurred.
03
Indicate the type of transfusion that was administered (e.g., red blood cells, platelets).
04
Describe the signs and symptoms of the reaction observed in the patient.
05
Include vital signs and any changes noted during the reaction.
06
Document the immediate actions taken in response to the reaction.
07
List any medications administered as part of the treatment.
08
Provide details of the personnel notified about the reaction.
09
Complete the section regarding the patient's past transfusion history.
10
Sign and date the form before submitting it to the appropriate department.
Who needs Transfusion Reaction Investigation Form (CM105)?
01
The Transfusion Reaction Investigation Form (CM105) is needed by healthcare professionals involved in transfusion therapy, including nurses, doctors, and blood bank staff, to document and report any adverse reactions to blood transfusions.
Fill
form
: Try Risk Free






People Also Ask about
What is the first check performed in the investigation of a transfusion reaction?
When a transfusion reaction is suspected, the transfusion should be immediately stopped, and the intravenous line should be kept open using appropriate fluids (usually 0.9% saline). A clerical check should be performed by examining the product bag and confirming the patient's identification.
How to investigate a transfusion reaction?
In acute hemolytic reactions, the workup includes the following: Visual inspection of the recipient's plasma and . Retyping of donor and recipient red blood cells (RBCs) Direct antiglobulin (Coombs) testing.
How to document a blood transfusion reaction?
Document the time and date of the reaction, type and amount of infused blood or blood product, time you started the transfusion, and time you stopped it.
What should you do if you suspect a transfusion reaction?
Some symptoms may resolve with little or no treatment. However, respiratory distress, high fever, hypotension, and hemoglobinuria may indicate a more serious reaction. All cases of suspected reactions should prompt immediate discontinuation of the transfusion and notification of the blood bank and treating clinician.
How do you investigate a transfusion reaction?
Routine blood tests for Transfusion Reaction investigations; 6ml EDTA (pink) for repeat cross match and group and screen. 6ml clotted sample for U&E's, LFT's, LDH, Haptoglobin. 4ml EDTA (purple) for Full Blood Count. 2.7ml citrate (blue) for coagulation screen. sample (to check for haemoglobin / haematuria)
What are the 3 R's of blood transfusion reactions?
The three "R"s of blood transfusion in 2020; routine, reliable and robust.
How to test for transfusion reaction?
Blood samples from the recipient (person getting the transfusion) and from the donor must be tested to tell whether symptoms are being caused by a transfusion reaction.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is Transfusion Reaction Investigation Form (CM105)?
The Transfusion Reaction Investigation Form (CM105) is a standardized document used to report and investigate adverse reactions that may occur following a blood transfusion. It helps ensure patient safety and facilitates the tracking of transfusion-related complications.
Who is required to file Transfusion Reaction Investigation Form (CM105)?
The Transfusion Reaction Investigation Form (CM105) must be filed by healthcare professionals, such as physicians, nurses, or transfusion service personnel, who are involved in the patient care during or after a blood transfusion when an adverse reaction is suspected.
How to fill out Transfusion Reaction Investigation Form (CM105)?
To fill out the Transfusion Reaction Investigation Form (CM105), you must provide detailed information about the patient, transfusion details, the nature of the reaction, clinical observations, laboratory findings, and any interventions performed. It’s essential to complete all required fields accurately and in a timely manner.
What is the purpose of Transfusion Reaction Investigation Form (CM105)?
The purpose of the Transfusion Reaction Investigation Form (CM105) is to systematically collect information about transfusion reactions to identify their causes, improve transfusion practices, enhance patient safety, and contribute to data collection for quality improvement initiatives in transfusion medicine.
What information must be reported on Transfusion Reaction Investigation Form (CM105)?
The information that must be reported on the Transfusion Reaction Investigation Form (CM105) includes patient demographics, type of transfusion, volume of blood component transfused, time of transfusion, symptoms observed, onset time of the reaction, any relevant medical history, laboratory test results, and the actions taken in response to the reaction.
Fill out your transfusion reaction investigation form online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Transfusion Reaction Investigation Form is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.