Get the free Application for prevest clinical research award - Prevest Denpro ...
Show details
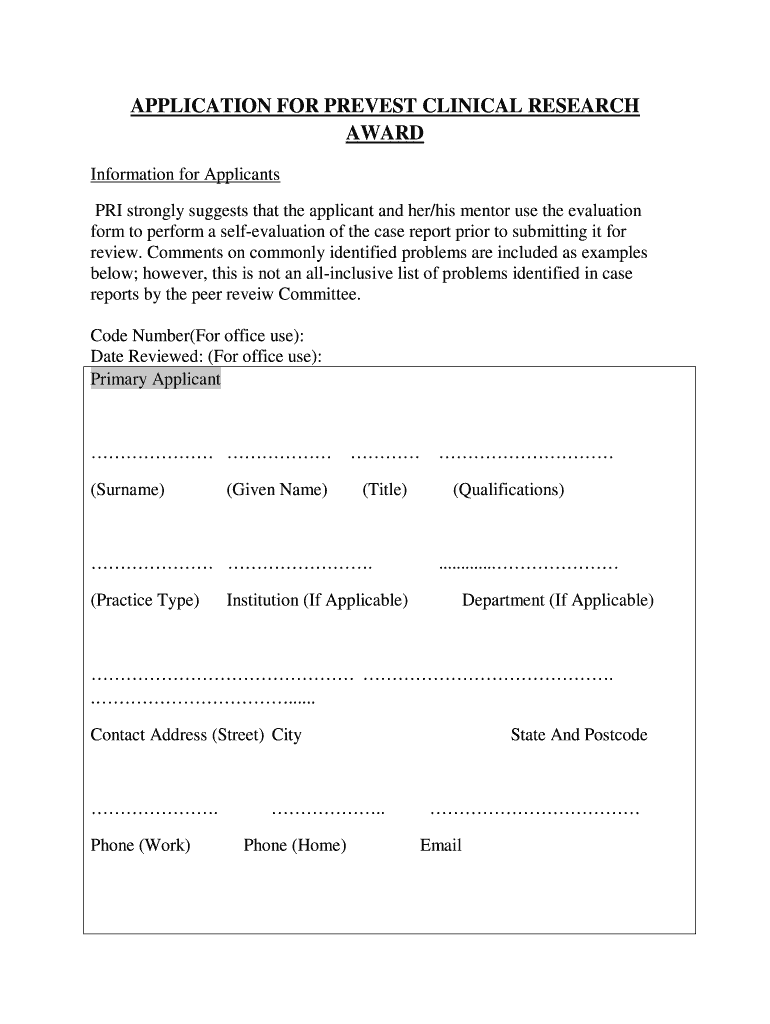
APPLICATION FOR PREVENT CLINICAL RESEARCH
AWARD
Information for Applicants
PRI strongly suggests that the applicant and her×his mentor use the evaluation
form to perform a self evaluation of the
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign application for prevest clinical

Edit your application for prevest clinical form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your application for prevest clinical form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit application for prevest clinical online
To use our professional PDF editor, follow these steps:
1
Create an account. Begin by choosing Start Free Trial and, if you are a new user, establish a profile.
2
Upload a document. Select Add New on your Dashboard and transfer a file into the system in one of the following ways: by uploading it from your device or importing from the cloud, web, or internal mail. Then, click Start editing.
3
Edit application for prevest clinical. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Get your file. Select the name of your file in the docs list and choose your preferred exporting method. You can download it as a PDF, save it in another format, send it by email, or transfer it to the cloud.
Dealing with documents is always simple with pdfFiller.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out application for prevest clinical

How to fill out application for prevest clinical:
01
Start by gathering all the required documents and information. This may include your personal details such as name, contact information, and address, as well as your educational background, work experience, and any relevant certifications or licenses.
02
Read through the application form carefully, ensuring that you understand all the sections and questions. If there are any specific instructions or guidelines provided, be sure to follow them closely.
03
Begin filling in the application form, providing accurate and up-to-date information. Double-check your entries for any errors or typos before moving on to the next section.
04
Pay attention to any additional documents or attachments that may be required. These could include a resume, cover letter, references, or any supporting documents related to your qualifications.
05
Take your time to thoroughly complete each section of the application. Provide clear and concise responses, and be as honest and detailed as possible.
06
Proofread your application once you have completed it. Look for any grammatical or spelling errors, and ensure that all the information provided is accurate.
07
If there are any specific submission guidelines mentioned, make sure to follow them. This could involve submitting the application online, mailing it, or dropping it off at a specific location.
Who needs an application for prevest clinical?
01
Individuals who are interested in pursuing a career or participating in a clinical trial at prevest clinical may need to fill out an application. This could include medical professionals, researchers, volunteers, or individuals seeking treatment.
02
Job seekers who are applying for positions within prevest clinical, such as research assistants, clinical coordinators, or laboratory technicians, may also need to complete an application.
03
Patients who wish to participate in clinical trials conducted by prevest clinical may be required to fill out an application to provide their medical history and qualifications for the study.
In conclusion, anyone interested in a career opportunity, research collaboration, or patient participation at prevest clinical may need to fill out an application. It is important to carefully follow the instructions, provide accurate information, and submit all required documents to increase the chances of a successful application.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I complete application for prevest clinical online?
Completing and signing application for prevest clinical online is easy with pdfFiller. It enables you to edit original PDF content, highlight, blackout, erase and type text anywhere on a page, legally eSign your form, and much more. Create your free account and manage professional documents on the web.
How do I edit application for prevest clinical online?
pdfFiller allows you to edit not only the content of your files, but also the quantity and sequence of the pages. Upload your application for prevest clinical to the editor and make adjustments in a matter of seconds. Text in PDFs may be blacked out, typed in, and erased using the editor. You may also include photos, sticky notes, and text boxes, among other things.
How can I fill out application for prevest clinical on an iOS device?
In order to fill out documents on your iOS device, install the pdfFiller app. Create an account or log in to an existing one if you have a subscription to the service. Once the registration process is complete, upload your application for prevest clinical. You now can take advantage of pdfFiller's advanced functionalities: adding fillable fields and eSigning documents, and accessing them from any device, wherever you are.
What is application for prevest clinical?
The application for prevest clinical is a form that must be submitted to request permission to conduct clinical trials for a new medical product.
Who is required to file application for prevest clinical?
Any organization or individual looking to conduct clinical trials for a new medical product is required to file the application for prevest clinical.
How to fill out application for prevest clinical?
The application for prevest clinical must be filled out completely and accurately with all relevant information regarding the clinical trials and the new medical product.
What is the purpose of application for prevest clinical?
The purpose of the application for prevest clinical is to obtain approval from regulatory authorities to conduct clinical trials for a new medical product.
What information must be reported on application for prevest clinical?
The application for prevest clinical must include detailed information about the new medical product, the proposed clinical trials, and supporting documentation to demonstrate safety and efficacy.
Fill out your application for prevest clinical online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Application For Prevest Clinical is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.


















