Get the free Rituximab Phase I-II Clinical Report - proofs - Opsoclonus ... - omsusa
Show details
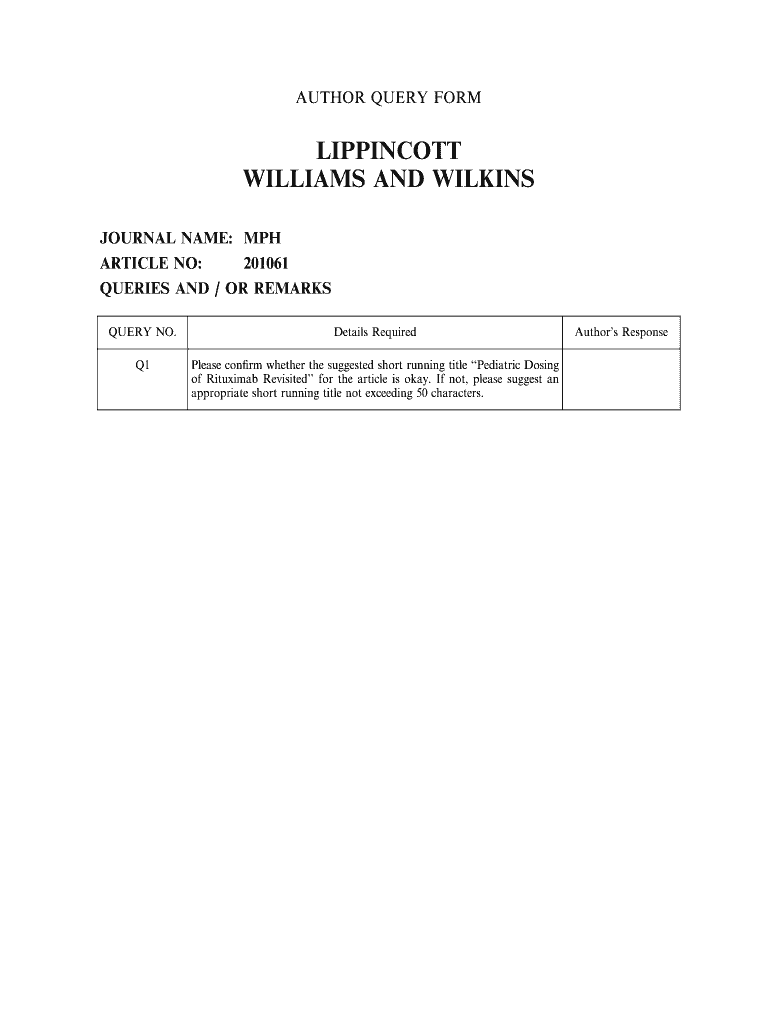
AUTHOR QUERY FORM LIPPINCOTT WILLIAMS AND WILKINS JOURNAL NAME: MPH ARTICLE NO: 201061 QUERIES AND / OR REMARKS QUERY NO. Details Required Q1 Please corm whether the suggested short running title
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign rituximab phase i-ii clinical

Edit your rituximab phase i-ii clinical form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your rituximab phase i-ii clinical form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit rituximab phase i-ii clinical online
Use the instructions below to start using our professional PDF editor:
1
Log in. Click Start Free Trial and create a profile if necessary.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit rituximab phase i-ii clinical. Text may be added and replaced, new objects can be included, pages can be rearranged, watermarks and page numbers can be added, and so on. When you're done editing, click Done and then go to the Documents tab to combine, divide, lock, or unlock the file.
4
Get your file. When you find your file in the docs list, click on its name and choose how you want to save it. To get the PDF, you can save it, send an email with it, or move it to the cloud.
The use of pdfFiller makes dealing with documents straightforward. Try it right now!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out rituximab phase i-ii clinical

How to fill out rituximab phase i-ii clinical:
01
Obtain the necessary forms and documentation required for the rituximab phase i-ii clinical trial. This may include informed consent forms, medical history questionnaires, and laboratory test results.
02
Familiarize yourself with the specific instructions and guidelines provided by the clinical trial organizers. It is important to understand the requirements and procedures involved in the rituximab phase i-ii clinical trial to ensure accurate and thorough completion of the forms.
03
Begin by filling out the informed consent form. This document typically outlines the purpose and objectives of the clinical trial, potential risks and benefits, and the participant's rights and responsibilities. Read it carefully, ask any questions you may have, and sign it only if you fully understand and agree to participate.
04
Proceed to complete the medical history questionnaire. This form collects detailed information about your health, including any underlying medical conditions, prior treatments or surgeries, and current medications. Provide accurate and comprehensive responses, as these details will be essential for evaluating your eligibility for the rituximab phase i-ii clinical trial.
05
Follow any specific instructions regarding laboratory tests. The clinical trial organizers may request specific blood tests or other diagnostic procedures to assess your baseline health status before starting rituximab treatment. Schedule these tests as instructed and ensure that the results are documented correctly on the appropriate forms.
06
If applicable, provide details about any previous rituximab treatments you have received. This may include information about the dosage, duration, and outcomes of previous rituximab therapies. Such information allows researchers to better understand any potential impacts on your current response to the drug.
07
Submit the completed forms and any supporting documentation to the clinical trial organizers. It is essential to provide all necessary information accurately and in a timely manner to ensure your eligibility and smooth participation in the rituximab phase i-ii clinical trial.
Who needs rituximab phase i-ii clinical:
01
Individuals diagnosed with certain types of cancer that have not responded adequately to standard treatments may benefit from participating in a rituximab phase i-ii clinical trial. These trials aim to evaluate the safety, efficacy, and optimal dosage of rituximab in specific cancer populations.
02
Patients who have exhausted other treatment options without achieving satisfactory results may be eligible for rituximab phase i-ii clinical trials. These trials often target patients with advanced or refractory cancers, offering a potential alternative or additional therapy to explore.
03
Individuals who are willing to contribute to medical research and help advance scientific knowledge in the field of cancer treatment may choose to participate in rituximab phase i-ii clinical trials. By volunteering, they actively support the development of new therapies and contribute to potential breakthroughs in cancer care.
Note: Before considering participation in a rituximab phase i-ii clinical trial, it is vital to consult with your healthcare provider to determine whether it is an appropriate and suitable option for your specific medical condition and circumstances.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I edit rituximab phase i-ii clinical in Chrome?
Adding the pdfFiller Google Chrome Extension to your web browser will allow you to start editing rituximab phase i-ii clinical and other documents right away when you search for them on a Google page. People who use Chrome can use the service to make changes to their files while they are on the Chrome browser. pdfFiller lets you make fillable documents and make changes to existing PDFs from any internet-connected device.
Can I create an eSignature for the rituximab phase i-ii clinical in Gmail?
Create your eSignature using pdfFiller and then eSign your rituximab phase i-ii clinical immediately from your email with pdfFiller's Gmail add-on. To keep your signatures and signed papers, you must create an account.
How do I fill out rituximab phase i-ii clinical using my mobile device?
Use the pdfFiller mobile app to fill out and sign rituximab phase i-ii clinical on your phone or tablet. Visit our website to learn more about our mobile apps, how they work, and how to get started.
What is rituximab phase i-ii clinical?
Rituximab phase I-II clinical trial is a study conducted on a small group of patients to evaluate the safety and effectiveness of rituximab in treating specific medical conditions.
Who is required to file rituximab phase i-ii clinical?
The pharmaceutical company or research institution conducting the clinical trial is required to file rituximab phase I-II clinical trial.
How to fill out rituximab phase i-ii clinical?
To fill out rituximab phase I-II clinical trial, researchers need to provide detailed information about the study design, patient population, treatment protocol, and data analysis plan.
What is the purpose of rituximab phase i-ii clinical?
The purpose of rituximab phase I-II clinical trial is to gather preliminary data on the safety and efficacy of rituximab in a controlled setting before proceeding to larger scale phase III clinical trials.
What information must be reported on rituximab phase i-ii clinical?
Information that must be reported on rituximab phase I-II clinical trial includes patient demographics, adverse events, treatment response rates, and any other relevant clinical outcomes.
Fill out your rituximab phase i-ii clinical online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Rituximab Phase I-Ii Clinical is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.