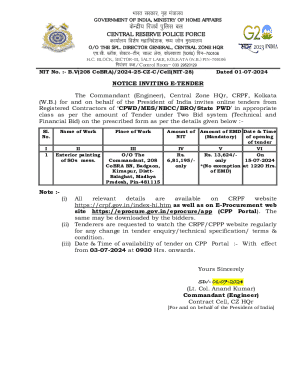
Get the free Acid Base Chemistry - faculty sdmiramar
Show details
This document is a practice exam focusing on topics from acid-base chemistry, including calculations, definitions, and relationships of acids, bases, pH, pOH, and various associated equations and
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign acid base chemistry

Edit your acid base chemistry form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your acid base chemistry form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing acid base chemistry online
To use our professional PDF editor, follow these steps:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit acid base chemistry. Replace text, adding objects, rearranging pages, and more. Then select the Documents tab to combine, divide, lock or unlock the file.
4
Save your file. Choose it from the list of records. Then, shift the pointer to the right toolbar and select one of the several exporting methods: save it in multiple formats, download it as a PDF, email it, or save it to the cloud.
pdfFiller makes working with documents easier than you could ever imagine. Create an account to find out for yourself how it works!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out acid base chemistry

How to fill out Acid Base Chemistry
01
Understand the concept of pH and how it relates to acidity and basicity.
02
Learn about the Arrhenius, Brønsted-Lowry, and Lewis definitions of acids and bases.
03
Familiarize yourself with acid-base neutralization reactions and how to balance them.
04
Practice using pH indicators and titration techniques to measure acidity and basicity.
05
Study the role of buffers in maintaining pH in solutions.
06
Review strong vs weak acids and bases, and their dissociation in water.
07
Apply the concepts to real-life scenarios such as biochemistry, environmental science, and industrial processes.
Who needs Acid Base Chemistry?
01
Students studying chemistry, particularly high school and college-level courses.
02
Professionals working in fields such as medicine, pharmaceuticals, and environmental science.
03
Researchers conducting experiments involving chemical reactions and solutions.
04
Anyone interested in understanding everyday products like cleaning agents or food chemistry.
Fill
form
: Try Risk Free






People Also Ask about
What are the 7 types of bases?
Broadly, bases are classified into various categories, i.e., strong base, weak base, super base, solid base, neutral base, etc. Strong bases: A species that can remove a proton (H+) from a molecule in an acid-base reaction is termed a strong base. Weak bases: Superbase: Neutral Base: Solid Base:
What are the 7 main strong acids?
There are 7 strong acids: chloric acid, hydrobromic acid, hydrochloric acid, hydroiodic acid, nitric acid, perchloric acid, and sulfuric acid.
What is an acid and a base in English?
The chemical difference between acids and bases is that acids produce hydrogen ions and bases accept hydrogen ions. A base is a substance that neutralises acids. When bases are added to water, they split to form hydroxide ions, written as OH-. We call a base that has been added to water an alkaline solution.
What are the 7 properties of bases?
Bases have properties that mostly contrast with those of acids. Aqueous solutions of bases are also electrolytes. Bases often have a bitter taste and are found in foods less frequently than acids. Bases also change the color of indicators. Bases do not react with metals in the way that acids do.
What are the 7 types of acids?
Examples of Acids AcidsFormulaUse/Source Hydrochloric acid HCl Stomach juices, cleaning solutions Nitric acid HNO3 fertilizers, plastics, dyes Citric acid C6 H8 O7 Lemons, oranges, dietary supplements of vitamin C Acetic acid HC2 H3 O2 Vinegar which is used in cleaning and various food preparations3 more rows
What is an acid and base in chemistry?
The earliest definition of acids and bases is Arrhenius's definition which states that: An acid is a substance that forms hydrogen ions H+ when dissolved in water, and. A base is a substance that forms hydroxide ions OH- when dissolved in water.
What are the 7 properties of acid?
Acids Aqueous solutions of acids are electrolytes, meaning that they conduct electrical current. Acids have a sour taste. Acids change the color of certain acid-base indicates. Acids react with active metals to yield hydrogen gas. Acids react with bases to produce a salt compound and water.
What are 7 examples of chemical properties?
10 examples of chemical properties include flammability, toxicity, solubility, heat from combustion, radioactivity, types of chemical bonds formed, coordination number, oxidization states, and acidity or basicity.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is Acid Base Chemistry?
Acid Base Chemistry is the study of the properties, behaviors, and reactions of acids and bases, which are substances that can donate and accept protons (H+ ions) respectively. It encompasses the principles of pH, acidity, basicity, and the interactions between acids and bases.
Who is required to file Acid Base Chemistry?
Generally, individuals or organizations engaged in chemical manufacturing, research, or educational institutions that involve experiments or analysis related to acids and bases are required to file Acid Base Chemistry.
How to fill out Acid Base Chemistry?
Filling out Acid Base Chemistry typically involves documenting the specific acids and bases used in experiments, their concentrations, pH levels, and any reactions observed. It may require following a standardized format or guidelines set by regulatory agencies.
What is the purpose of Acid Base Chemistry?
The purpose of Acid Base Chemistry is to understand chemical behavior in terms of proton transfer, predict the outcome of chemical reactions, determine the pH of solutions, and apply this knowledge in various scientific and industrial applications.
What information must be reported on Acid Base Chemistry?
Information that must be reported on Acid Base Chemistry includes the types of acids and bases used, their concentrations, pH values, reaction products, and any safety data relevant to handling these substances.
Fill out your acid base chemistry online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Acid Base Chemistry is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.