Get the free APPLICATION FOR INVESTIGATIONAL HUMAN USE - UC Office of ... - researchcompliance uc
Show details
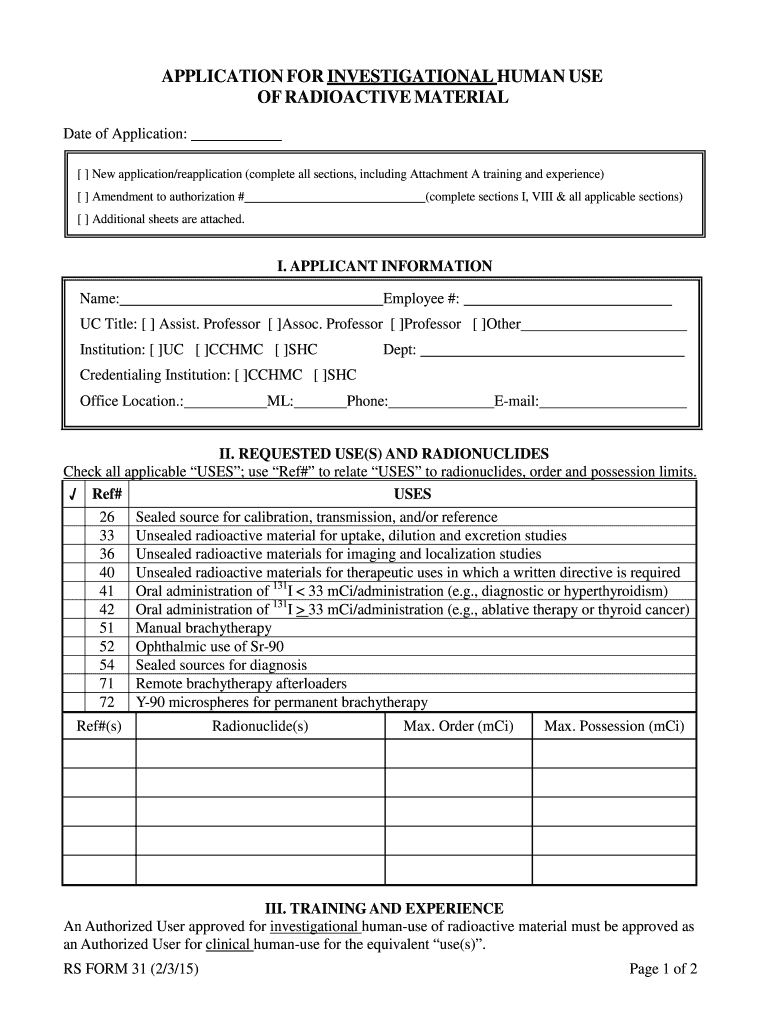
APPLICATION FOR INVESTIGATIONAL HUMAN USE OF RADIOACTIVE MATERIAL Date of Application: New application×reapplication (complete all sections, including Attachment A training and experience) Amendment
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign application for investigational human

Edit your application for investigational human form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your application for investigational human form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit application for investigational human online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit application for investigational human. Text may be added and replaced, new objects can be included, pages can be rearranged, watermarks and page numbers can be added, and so on. When you're done editing, click Done and then go to the Documents tab to combine, divide, lock, or unlock the file.
4
Save your file. Select it from your records list. Then, click the right toolbar and select one of the various exporting options: save in numerous formats, download as PDF, email, or cloud.
pdfFiller makes working with documents easier than you could ever imagine. Register for an account and see for yourself!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out application for investigational human

How to fill out an application for investigational human:
01
Start by gathering all the necessary information and documents required for the application. This typically includes personal details of the individual being investigated, such as their name, contact information, date of birth, and social security number.
02
Next, provide information about the purpose and scope of the investigation. Explain the reason for conducting the investigation, whether it is for scientific research, medical trials, or any other relevant purpose.
03
Include any relevant background information about the individual being investigated. This may include their medical history, previous participation in similar studies, and any potential risks or challenges associated with the investigation.
04
Specify the procedures and methods that will be used during the investigation. Detail the specific tests, treatments, or interventions that will be administered and explain their potential impact on the individual.
05
Outline the timeline and duration of the investigation. Provide a clear schedule indicating the start and end dates of the study, as well as any follow-up or monitoring periods.
06
Discuss the potential benefits and risks associated with the investigation. Highlight any potential benefits the individual may receive from participating in the study, as well as any known risks or side effects.
07
Include any required consent forms or waivers. Ensure that the individual understands the purpose and nature of the investigation, and provide them with the necessary documentation to give their informed consent.
08
Review and proofread the application thoroughly before submitting it. Ensure that all sections are filled out accurately and that all supporting documents are included.
Who needs an application for investigational human?
01
Researchers conducting scientific studies or medical trials may require an application for investigational human subjects to gather data and information for their research.
02
Pharmaceutical companies or medical institutions developing new drugs or treatments may need an application for investigational human subjects to test the efficacy and safety of their products.
03
Government agencies or regulatory bodies overseeing research or medical experiments may require an application for investigational human subjects to ensure that ethical guidelines and regulations are followed.
In conclusion, anyone involved in conducting scientific studies, medical trials, or research involving human subjects may need to fill out an application for investigational humans. This ensures that proper protocols and regulations are followed, and that the rights and well-being of the individuals involved are protected.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I edit application for investigational human from Google Drive?
pdfFiller and Google Docs can be used together to make your documents easier to work with and to make fillable forms right in your Google Drive. The integration will let you make, change, and sign documents, like application for investigational human, without leaving Google Drive. Add pdfFiller's features to Google Drive, and you'll be able to do more with your paperwork on any internet-connected device.
How do I make edits in application for investigational human without leaving Chrome?
Install the pdfFiller Google Chrome Extension to edit application for investigational human and other documents straight from Google search results. When reading documents in Chrome, you may edit them. Create fillable PDFs and update existing PDFs using pdfFiller.
Can I create an electronic signature for signing my application for investigational human in Gmail?
You can easily create your eSignature with pdfFiller and then eSign your application for investigational human directly from your inbox with the help of pdfFiller’s add-on for Gmail. Please note that you must register for an account in order to save your signatures and signed documents.
What is application for investigational human?
The application for investigational human is a formal request submitted to regulatory authorities to conduct clinical trials on humans with investigational products.
Who is required to file application for investigational human?
Any individual or organization planning to conduct clinical trials on humans with investigational products is required to file an application for investigational human.
How to fill out application for investigational human?
The application for investigational human must be filled out according to the guidelines provided by regulatory authorities, including details about the investigational product, trial design, and safety measures.
What is the purpose of application for investigational human?
The purpose of the application for investigational human is to obtain approval from regulatory authorities to conduct clinical trials on humans with investigational products, ensuring the safety and efficacy of the products.
What information must be reported on application for investigational human?
The application for investigational human must include detailed information about the investigational product, trial protocol, informed consent process, safety monitoring plans, and qualifications of investigators.
Fill out your application for investigational human online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Application For Investigational Human is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.



















