Get the free REQUEST FOR IRB REVIEW OF HUMANITARIAN USE DEVICE (HUD) CONTINUING REVIEW FORM
Show details
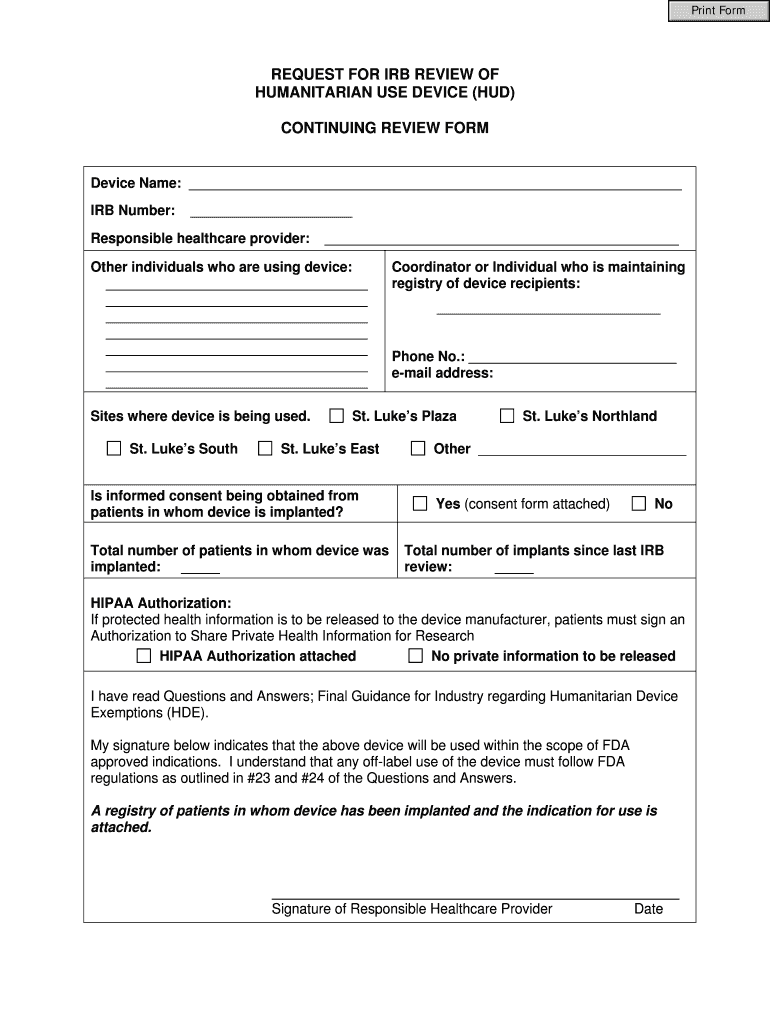
This document is used to request IRB review for the continuation of a Humanitarian Use Device (HUD) study, including details about the device, healthcare providers, and patient information.
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign request for irb review

Edit your request for irb review form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your request for irb review form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing request for irb review online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Log into your account. It's time to start your free trial.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit request for irb review. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
With pdfFiller, it's always easy to work with documents.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out request for irb review

How to fill out REQUEST FOR IRB REVIEW OF HUMANITARIAN USE DEVICE (HUD) CONTINUING REVIEW FORM
01
Obtain the REQUEST FOR IRB REVIEW OF HUMANITARIAN USE DEVICE (HUD) CONTINUING REVIEW FORM from your institution's IRB website or office.
02
Fill out the project title and investigator information accurately.
03
Provide a detailed description of the humanitarian use device, including the intended use and patient population.
04
Indicate the number of patients treated with the device since the last review.
05
Include any adverse events or problems that have occurred during the use of the device.
06
Attach any relevant supporting documents, such as data collection forms, consent forms, and research updates.
07
Review the completed form for accuracy and completeness.
08
Submit the form to the IRB office according to your institution's guidelines.
Who needs REQUEST FOR IRB REVIEW OF HUMANITARIAN USE DEVICE (HUD) CONTINUING REVIEW FORM?
01
Researchers conducting studies involving a humanitarian use device.
02
Medical professionals using or overseeing the use of the device in a clinical setting.
03
Institutions seeking to ensure compliance with ethical standards regarding human subject research.
Fill
form
: Try Risk Free






People Also Ask about
What is the humanitarian device exemption?
Humanitarian Device Exemption (HDE): a marketing application for an HUD (Section 520(m) of the Federal Food, Drug, and Cosmetic Act (FD&C Act)). An HDE is exempt from the effectiveness requirements of Sections 514 and 515 of the FD&C Act and is subject to certain profit and use restrictions.
What does an investigation to collect data on a new indication of a significant risk HUD require?
Which of the following statements is true regarding IRB approval of clinical use of a HUD in a healthcare facility? Since a HUD is a marketed device, the regulations do not require IRB approval for clinical use. The clinician obtains initial IRB approval and ensures continuing review approval by the IRB.
Which of the following statements is true regarding IRB approval of clinical use of a HUD?
The provisions for obtaining an HDE are: The device is designed to treat or diagnose a disease or condition that affects fewer than 8,000 individuals per year in the U.S. The device is not available otherwise, and there is no comparable device available to treat or diagnose the disease or condition; and.
What does an investigation to collect data on a new indication of significant risk HUD require?
An HDE is a type of Pre-Market Approval (PMA) that allows the FDA to grant an exemption from the effectiveness requirements of the PMA regulations. Devices approved with an HDE are referred to as a Humanitarian Use Devices (HUD).
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is REQUEST FOR IRB REVIEW OF HUMANITARIAN USE DEVICE (HUD) CONTINUING REVIEW FORM?
The REQUEST FOR IRB REVIEW OF HUMANITARIAN USE DEVICE (HUD) CONTINUING REVIEW FORM is a document that must be submitted to an Institutional Review Board (IRB) for the ongoing review and oversight of a humanitarian use device that is being used in clinical settings. It ensures compliance with ethical standards and regulatory requirements.
Who is required to file REQUEST FOR IRB REVIEW OF HUMANITARIAN USE DEVICE (HUD) CONTINUING REVIEW FORM?
The form must be filed by the sponsor or principal investigator of the study involving the humanitarian use device. It is required for any ongoing clinical investigation where a HUD is being utilized.
How to fill out REQUEST FOR IRB REVIEW OF HUMANITARIAN USE DEVICE (HUD) CONTINUING REVIEW FORM?
To fill out the form, the investigator must provide detailed information regarding the ongoing use of the HUD, including patient outcomes, any adverse events, data on device performance, and updates on ongoing research activities. Accurate and thorough documentation is crucial for IRB review.
What is the purpose of REQUEST FOR IRB REVIEW OF HUMANITARIAN USE DEVICE (HUD) CONTINUING REVIEW FORM?
The purpose of the form is to facilitate the continuing review process of the HUD to ensure that it is being used ethically and safely in clinical settings. It helps the IRB assess whether the benefits to patients outweigh any potential risks associated with the device.
What information must be reported on REQUEST FOR IRB REVIEW OF HUMANITARIAN USE DEVICE (HUD) CONTINUING REVIEW FORM?
The form must include information such as patient enrollment numbers, adverse events or complications, data on device effectiveness, any modifications made to the device or protocol, and submission of any relevant literature related to the HUD.
Fill out your request for irb review online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Request For Irb Review is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















