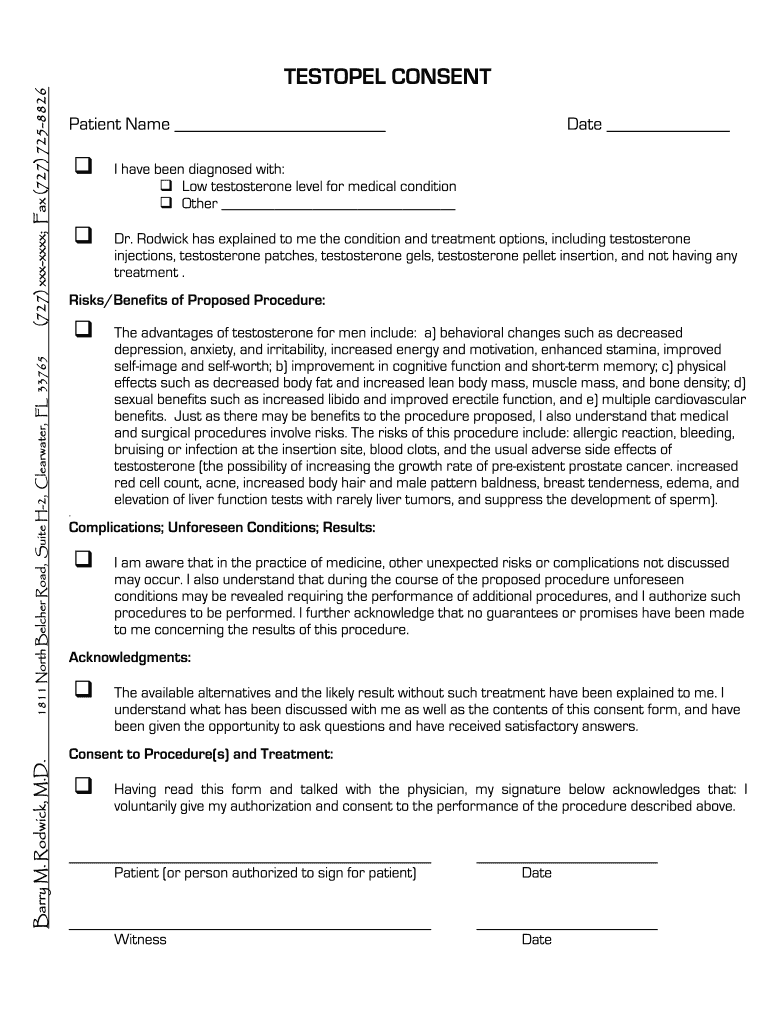
Barry M Rodwick Testopel Consent free printable template
Get, Create, Make and Sign m rodwick testopel consent template form



How to edit testopel consent form online
Uncompromising security for your PDF editing and eSignature needs
How to fill out rodwick testopel form

How to fill out Barry M. Rodwick Testopel Consent
Who needs Barry M. Rodwick Testopel Consent?
Video instructions and help with filling out and completing rodwick testopel
Instructions and Help about rodwick testopel form
Hello this video is to write guidance and how to create consent documents for your research study first we'll discuss the differences between different types of consent then we'll go through the necessary elements of consent and give you tips on information you'll need to include you can find more information at the OSU IRB website which appears below you can also find a copy of the consent templates and further guidance on the website why consent the purpose of consent is to help participants make an informed decision about whether to participate in your research study participants have the right to know what they're getting into by participating in your study they should know upfront what the time commitment will be and what the general procedures will be they should have a clear understanding of the risks and benefits of participating in the research it should also be clear who participants should contact if they have questions before or after the study the consent form is the standard document used to obtain consent participants are typically given a hard copy of the form and asked to read and sign the document a waiver of consent documentation is used when the IRB determines that you do not need to have a participant signature to indicate that they were consented the information included in the consent process is typically similar to the consent form for example in an online study they may click a button to indicate consent rather than providing a signature in this case you would want to seek a waiver of consent documentation a waiver of consent is granted when an IRB determines that it is not necessary to consent participants although rare these waivers can be granted for situations such as a study in which you're observing public behavior an alteration of consent is granted when the IRB determines that the study could not be conducted in a valid manner if participants knew all the details of the research before the study for example or if your design uses some form of deception you apply for an alteration of consent after the study you should provide additional information through debriefing and give participants the opportunity to withdraw their data from the study to get started we recommend downloading the consent template from the OSU IRB website due to federal state and university policies as well as changes in research practices the template changes over time it's helpful to get the most up-to-date document from the website because old consent forms you've used for other studies may be out of date after you've downloaded the template we strongly recommend not removing the default template language this information is there because it reflects mandatory elements of consent that must be communicated to participants a final reminder about language as you're filling out your consent form with information about your study think about your intended audience try to use common language that your participants will understand avoid jargon and...






People Also Ask about
Does Medicare pay for S0189?
Is TESTOPEL covered by Medicare?
Is testosterone pellet therapy FDA approved?
Why are BioTE pellets not FDA approved?
What is the procedure for TESTOPEL?
Why are testosterone pellets not FDA approved?
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I modify rodwick testopel form without leaving Google Drive?
How can I send rodwick testopel form for eSignature?
How do I fill out the rodwick testopel form form on my smartphone?
What is Barry M. Rodwick Testopel Consent?
Who is required to file Barry M. Rodwick Testopel Consent?
How to fill out Barry M. Rodwick Testopel Consent?
What is the purpose of Barry M. Rodwick Testopel Consent?
What information must be reported on Barry M. Rodwick Testopel Consent?
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.