Get the free IRB Policy 13: Informed Consent
Show details
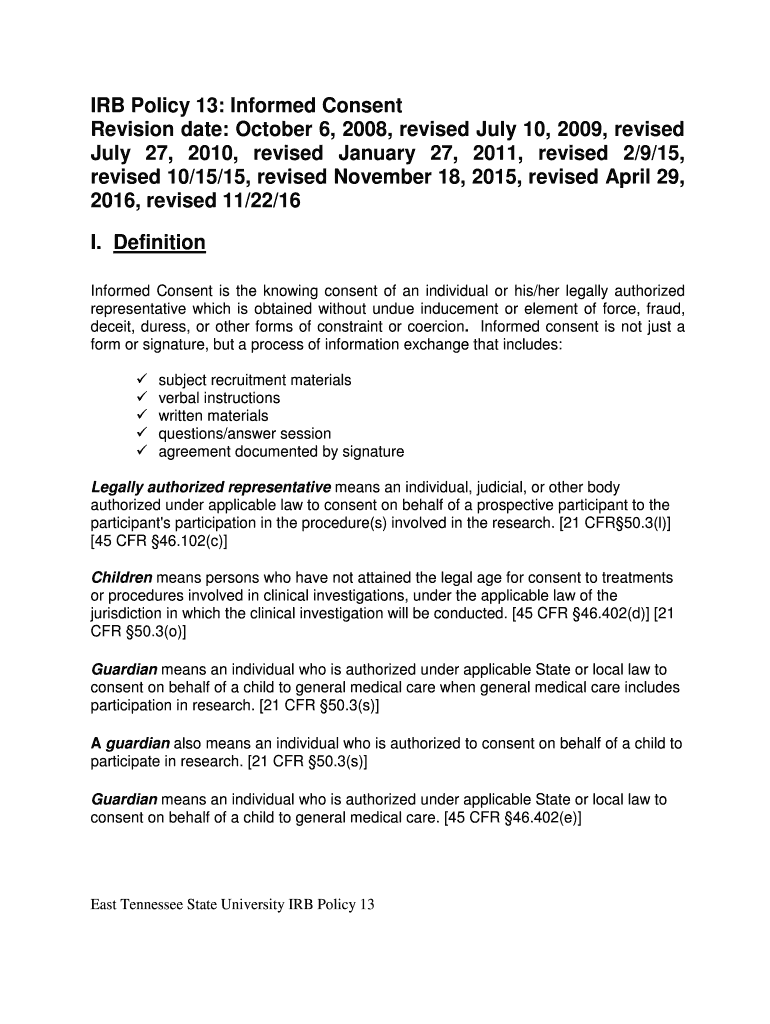
IRB Policy 13: Informed Consent
Revision date: October 6, 2008, revised July 10, 2009, revised
July 27, 2010, revised January 27, 2011, revised 2×9/15,
revised 10×15/15, revised November 18, 2015,
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign irb policy 13 informed

Edit your irb policy 13 informed form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your irb policy 13 informed form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit irb policy 13 informed online
To use the services of a skilled PDF editor, follow these steps below:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit irb policy 13 informed. Add and change text, add new objects, move pages, add watermarks and page numbers, and more. Then click Done when you're done editing and go to the Documents tab to merge or split the file. If you want to lock or unlock the file, click the lock or unlock button.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
With pdfFiller, dealing with documents is always straightforward. Try it right now!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out irb policy 13 informed

01
Start by carefully reading IRB Policy 13 to familiarize yourself with its requirements. Pay attention to any specific instructions or guidelines mentioned in the policy.
02
Identify if your research involves human subjects and if it falls under the jurisdiction of IRB Policy 13. This policy typically covers research that involves informed consent from human subjects.
03
Determine if you are the principal investigator or a member of the research team responsible for filling out the IRB Policy 13 informed consent form. Usually, the principal investigator takes on this responsibility.
04
Gather all necessary information and materials required to complete the informed consent form. This may include details about the study objectives, procedures, potential risks and benefits, confidentiality measures, and any compensation offered to participants.
05
Follow the specific format and layout provided by your institution's IRB office for the informed consent form. Pay attention to the sections that need to be filled out, such as participant information, study information, risks and benefits, and signatures.
06
Clearly and thoroughly explain the study to potential participants in a language that they can understand. Include all relevant information that they need to make an informed decision about participating in the research.
07
Ensure that the informed consent form includes provisions for participant consent, voluntary participation, and the freedom to withdraw from the study at any time.
08
Seek assistance or guidance from the IRB office or experienced researchers if you need clarification or help in completing the form accurately.
09
Once the form is completed, review it carefully for accuracy and completeness before submitting it to the IRB for review and approval.
10
Remember to maintain a copy of the informed consent form and any accompanying documents for your records as well as for future reference.
Who needs IRB Policy 13 informed?
01
Researchers, including principal investigators and their research teams, who are conducting studies that involve human subjects and require informed consent fall under the scope of IRB Policy 13. This policy ensures that ethical considerations, participant rights, and regulatory requirements are met during the research process.
02
Institutions or organizations that have research programs or departments, particularly those involved in human subjects research, are responsible for adhering to IRB policies, including Policy 13. These institutions typically have designated IRB offices or committees that oversee the process of obtaining informed consent and ensuring compliance with ethical standards.
03
Participants or potential subjects involved in studies that require informed consent from human subjects may also benefit from being aware of IRB Policy 13 informed. Understanding this policy helps participants make informed decisions about whether to participate in a research study and ensures their rights and well-being are protected throughout the research process.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I manage my irb policy 13 informed directly from Gmail?
The pdfFiller Gmail add-on lets you create, modify, fill out, and sign irb policy 13 informed and other documents directly in your email. Click here to get pdfFiller for Gmail. Eliminate tedious procedures and handle papers and eSignatures easily.
Can I sign the irb policy 13 informed electronically in Chrome?
You can. With pdfFiller, you get a strong e-signature solution built right into your Chrome browser. Using our addon, you may produce a legally enforceable eSignature by typing, sketching, or photographing it. Choose your preferred method and eSign in minutes.
How do I edit irb policy 13 informed on an iOS device?
Use the pdfFiller app for iOS to make, edit, and share irb policy 13 informed from your phone. Apple's store will have it up and running in no time. It's possible to get a free trial and choose a subscription plan that fits your needs.
What is irb policy 13 informed?
IRB Policy 13 informed refers to the policy regarding reporting of any information related to research studies or projects that involve human subjects.
Who is required to file irb policy 13 informed?
Researchers and investigators conducting research studies or projects involving human subjects are required to file IRB Policy 13 informed.
How to fill out irb policy 13 informed?
To fill out IRB Policy 13 informed, researchers need to provide all relevant and required information about their research study or project involving human subjects.
What is the purpose of irb policy 13 informed?
The purpose of IRB Policy 13 informed is to ensure transparency and compliance in research studies or projects involving human subjects.
What information must be reported on irb policy 13 informed?
Researchers must report details about their research study, including the purpose, methodology, risks involved, and measures taken to protect human subjects.
Fill out your irb policy 13 informed online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Irb Policy 13 Informed is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.