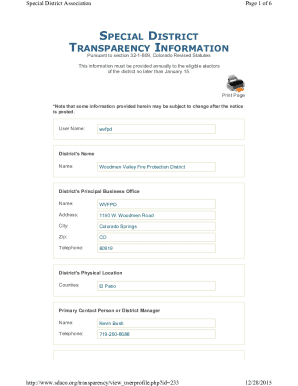
Get the free MRP & DCP step by step instructions how to apply and how the procedures are conducted
Show details
This document provides detailed instructions on how to apply for Marketing Authorization (MA) through the Mutual Recognition Procedure (MRP) and Decentralised Procedure (DCP), outlining the necessary
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign mrp dcp step by

Edit your mrp dcp step by form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your mrp dcp step by form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit mrp dcp step by online
To use our professional PDF editor, follow these steps:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit mrp dcp step by. Text may be added and replaced, new objects can be included, pages can be rearranged, watermarks and page numbers can be added, and so on. When you're done editing, click Done and then go to the Documents tab to combine, divide, lock, or unlock the file.
4
Get your file. Select the name of your file in the docs list and choose your preferred exporting method. You can download it as a PDF, save it in another format, send it by email, or transfer it to the cloud.
With pdfFiller, it's always easy to work with documents. Check it out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out mrp dcp step by

How to fill out MRP & DCP step by step instructions how to apply and how the procedures are conducted
01
Gather all required documents and information including personal identification, financial records, and any other relevant data.
02
Visit the official website or designated office for MRP & DCP applications.
03
Complete the application form by filling in all necessary details accurately.
04
Review the application for any errors or missing information before submission.
05
Pay any required application fees through the specified payment method.
06
Submit the completed application form along with supporting documents either online or in-person.
07
Keep a copy of the submitted application and any receipts for future reference.
08
Await confirmation of receipt from the relevant authority and follow up if necessary.
Who needs MRP & DCP step by step instructions how to apply and how the procedures are conducted?
01
Individuals seeking to obtain or renew their MRP & DCP identification.
02
Organizations or entities responsible for processing applications for MRP & DCP.
03
Legal guardians applying on behalf of minors for MRP & DCP.
04
Travelers who require MRP & DCP for international travel purposes.
Fill
form
: Try Risk Free






People Also Ask about
What is the DCP procedure for MRP?
What is the Decentralised procedure? The decentralised procedure ( DCP ) is a European authorisation route resulting in a mutually recognised product ( MRP ). The difference between MRP and DCP is that a product must already be authorised in at least one Member State on a national basis in order for MRP to be used.
What is MRP DCP?
The decentralised procedure ( DCP ) is a European authorisation route resulting in a mutually recognised product ( MRP ). The difference between MRP and DCP is that a product must already be authorised in at least one Member State on a national basis in order for MRP to be used.
What is the timeline of DCP procedure?
Decentralised Procedure ( DCP ) / Mutual Recognition Procedure ( MRP ) At present there are two different procedures to apply for a marketing authorisation for the same medicinal product in the EU/EEA in more than one Member State: the Decentralised Procedure ( DCP )
What is the MRP procedure?
Timelines for Decentralised Procedure The Coordination Group then must resolve issues and finalize documentation by day 150. The finalized report with agreed documentation is submitted to all member states for national decision-making. The goal is the complete the DCP procedure within 210 days.
What is a DCP procedure in medical terms?
Decentralized Procedure - Overview The Decentralized Procedure (DCP) allows the applicant to submit their application to the Health Authority (HA) and seek authorization in multiple EU member states simultaneously.
What is the MRP procedure?
There are many different organizations involved in the authorization procedures for medicinal products. On the basis of a single application, the Decentralized Procedure (DCP) is one of three ways to acquire regulatory approval to market medicinal products in several member states of the European Economic Area.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is MRP & DCP step by step instructions how to apply and how the procedures are conducted?
MRP (Market Research Process) is a systematic approach to collecting and analyzing data about market trends and consumer preferences. DCP (Data Collection Procedure) refers to the specific methods and frameworks used to gather this data. To apply for MRP and DCP, one should identify the research objectives, choose appropriate research methods (surveys, interviews, etc.), design data collection tools, pilot test them, gather data, analyze results, and finally report findings. Procedures involve planning, execution, and evaluation phases ensuring that the research adheres to ethical standards.
Who is required to file MRP & DCP step by step instructions how to apply and how the procedures are conducted?
Individuals or organizations conducting market research requiring approval typically file MRP and DCP. This includes marketing agencies, academic researchers, government entities, and businesses wanting to understand consumer behavior. It's crucial for them to ensure compliance with relevant legal and ethical frameworks when conducting research.
How to fill out MRP & DCP step by step instructions how to apply and how the procedures are conducted?
To fill out MRP and DCP, you should start by stating your research objectives clearly. Next, choose the appropriate forms applicable to your research type. Outline the methods of data collection you plan to use. Detail the sample size and demographics you intend to study. Include timelines for data collection and analysis, and specify how you will ensure data confidentiality. Finally, finish by obtaining necessary approvals and signatures before proceeding.
What is the purpose of MRP & DCP step by step instructions how to apply and how the procedures are conducted?
The purpose of MRP is to provide a structured approach to understanding market dynamics and consumer needs, enabling data-driven decisions. DCP serves to standardize the methods of data collection, ensuring reliability and validity in the findings. Together, they aim to inform stakeholders about market potentials and guide strategic planning.
What information must be reported on MRP & DCP step by step instructions how to apply and how the procedures are conducted?
MRP and DCP reporting should include the research objectives, methodology, sampling design, data collection instruments, analysis techniques, key findings, and recommendations. It is also important to document ethical considerations, limitations of the study, and any adjustments made during the research process.
Fill out your mrp dcp step by online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Mrp Dcp Step By is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.