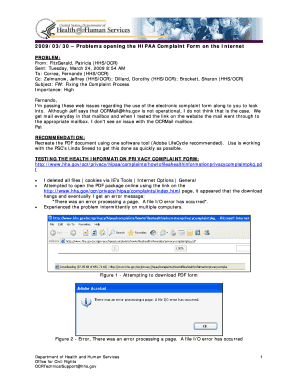
Get the free User-defined estimands
Show details
User defined estimandsIntroduction
Amos comes with the built-in ability to estimate all the quantities that you normally want to
estimate in a SEM analysis. This includes model parameters (like regression
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign user-defined estimands

Edit your user-defined estimands form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your user-defined estimands form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit user-defined estimands online
Follow the steps below to benefit from a competent PDF editor:
1
Log into your account. In case you're new, it's time to start your free trial.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit user-defined estimands. Replace text, adding objects, rearranging pages, and more. Then select the Documents tab to combine, divide, lock or unlock the file.
4
Get your file. Select the name of your file in the docs list and choose your preferred exporting method. You can download it as a PDF, save it in another format, send it by email, or transfer it to the cloud.
With pdfFiller, it's always easy to work with documents.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out user-defined estimands

How to fill out user-defined estimands:
01
Start by clearly defining the treatment or exposure variable of interest. This could be a specific medication, intervention, or any other factor that you want to assess the effect of.
02
Next, determine the outcome variable that you would like to measure. This could be a clinical endpoint, a biomarker, or any other measurable outcome that you believe is relevant to your research question.
03
Identify the population of interest. This involves specifying the characteristics of the individuals or groups that you want to study. This could include demographic information, medical history, or any other factors that may impact the treatment effect.
04
Consider potential time points for measurement. Determine when you would like to measure the outcome variable and how frequently it will be measured. This will help in capturing the treatment effect over time.
05
Select any relevant covariates that should be adjusted for in the analysis. These are variables that may confound the relationship between the treatment and outcome and should be taken into account to obtain accurate estimands.
06
Define the estimand. This is the precise quantity that you want to estimate, which represents the causal effect of the treatment on the outcome. Ensure that it aligns with your research question and is meaningful in the context of your study.
Who needs user-defined estimands?
01
Researchers and epidemiologists who want to conduct studies or clinical trials with specific research questions that need to be addressed by customized estimands.
02
Pharmaceutical companies and regulatory agencies that require a clear understanding and quantification of treatment effects to make informed decisions about drug approvals and use.
03
Policy-makers and decision-makers in healthcare who need reliable and comprehensive information about the effects of medical interventions to develop guidelines and allocate resources effectively.
By employing user-defined estimands and filling them out accurately, researchers can enhance the robustness and reliability of their findings, ensuring that the treatment effects are correctly estimated and understood. Similarly, decision-makers and stakeholders can benefit from user-defined estimands to make evidence-based decisions and policies for improved patient outcomes and public health.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I edit user-defined estimands from Google Drive?
It is possible to significantly enhance your document management and form preparation by combining pdfFiller with Google Docs. This will allow you to generate papers, amend them, and sign them straight from your Google Drive. Use the add-on to convert your user-defined estimands into a dynamic fillable form that can be managed and signed using any internet-connected device.
How can I edit user-defined estimands on a smartphone?
You may do so effortlessly with pdfFiller's iOS and Android apps, which are available in the Apple Store and Google Play Store, respectively. You may also obtain the program from our website: https://edit-pdf-ios-android.pdffiller.com/. Open the application, sign in, and begin editing user-defined estimands right away.
Can I edit user-defined estimands on an Android device?
You can. With the pdfFiller Android app, you can edit, sign, and distribute user-defined estimands from anywhere with an internet connection. Take use of the app's mobile capabilities.
What is user-defined estimands?
User-defined estimands are specific parameters that researchers define to measure the treatment effect in clinical trials.
Who is required to file user-defined estimands?
Researchers conducting clinical trials are required to file user-defined estimands.
How to fill out user-defined estimands?
User-defined estimands should be filled out by specifying the parameters, methods of estimation, and assumptions used.
What is the purpose of user-defined estimands?
The purpose of user-defined estimands is to provide a clear and pre-specified definition of the treatment effect to be estimated in a clinical trial.
What information must be reported on user-defined estimands?
Information such as the treatment effect parameter, the analysis population, and any sensitivity analyses must be reported on user-defined estimands.
Fill out your user-defined estimands online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

User-Defined Estimands is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.