Get the free ISO 13485 QMS Matrix
Show details
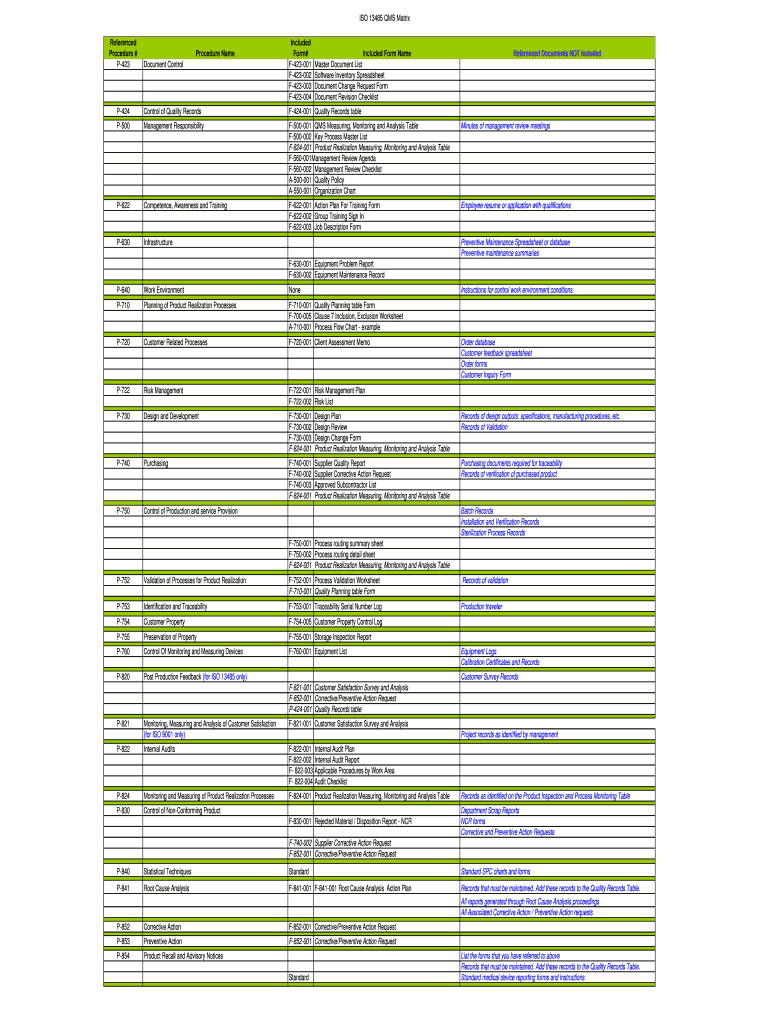
ISO 13485 RMS Matrix
Referenced
Procedure #
P423Procedure Name
Document ControlIncluded
Form#
F423001
F423002
F423003
F423004Included Form Name
Master Document List
Software Inventory Spreadsheet
Document
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign iso 13485 qms matrix

Edit your iso 13485 qms matrix form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your iso 13485 qms matrix form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit iso 13485 qms matrix online
In order to make advantage of the professional PDF editor, follow these steps:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit iso 13485 qms matrix. Replace text, adding objects, rearranging pages, and more. Then select the Documents tab to combine, divide, lock or unlock the file.
4
Save your file. Select it from your records list. Then, click the right toolbar and select one of the various exporting options: save in numerous formats, download as PDF, email, or cloud.
pdfFiller makes working with documents easier than you could ever imagine. Register for an account and see for yourself!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out iso 13485 qms matrix

How to fill out ISO 13485 QMS matrix:
01
Identify the relevant processes: Begin by understanding the processes within your organization that are related to the ISO 13485 Quality Management System (QMS). This may include areas such as management, design and development, purchasing, production, etc.
02
Define the parameters: For each process, establish the parameters that need to be documented in the QMS matrix. These parameters may include process inputs, outputs, resources utilized, responsibilities, performance indicators, etc.
03
Gather the required information: Collect all the necessary information for each parameter identified. This may involve conducting interviews, reviewing documentation, and observing the processes in action. Ensure that you have accurate and up-to-date information.
04
Create the matrix template: Design a matrix template that suits your organization's needs. This could be a simple spreadsheet with columns representing the processes and rows representing the parameters. Make it easy to understand and use.
05
Populate the matrix: Start completing the matrix by entering the relevant information for each parameter under the respective process. Include any additional notes or references that may be required.
06
Review and validate: Once you have filled out the matrix, review it carefully to ensure accuracy and completeness. Validate the information with the process owners and other relevant stakeholders to guarantee that it accurately reflects the organization's QMS.
07
Update and maintain: The ISO 13485 QMS matrix is a living document, so it is essential to keep it up to date. Regularly review and update the matrix as processes evolve or new ones are introduced. Ensure that the changes are communicated to all relevant personnel.
Who needs ISO 13485 QMS matrix?
01
Organizations seeking ISO 13485 certification: If your organization wants to obtain ISO 13485 certification, you will need to have a well-defined QMS matrix. It demonstrates your commitment to meeting the requirements of the standard and provides a clear overview of your processes.
02
Companies implementing quality management systems: Even if certification is not the immediate goal, organizations interested in enhancing their quality management practices can benefit from using an ISO 13485 QMS matrix. It helps structure and document processes, making it easier to identify areas for improvement and ensure compliance with regulatory requirements.
03
Quality managers and process owners: Quality managers and process owners within organizations that already have ISO 13485 certification or are in the process of implementing it can make use of the QMS matrix. It serves as a valuable tool for monitoring and managing process performance, allocating resources, and identifying opportunities for optimization.
In conclusion, the ISO 13485 QMS matrix is an essential tool for organizations implementing or seeking ISO 13485 certification. It helps in filling out the matrix by following specific steps, including identifying processes, defining parameters, gathering information, creating a matrix template, populating the matrix, reviewing and validating, and updating and maintaining it. Various stakeholders, including organizations seeking certification, companies implementing QMS, quality managers, and process owners, could benefit from using the ISO 13485 QMS matrix.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I edit iso 13485 qms matrix in Chrome?
Adding the pdfFiller Google Chrome Extension to your web browser will allow you to start editing iso 13485 qms matrix and other documents right away when you search for them on a Google page. People who use Chrome can use the service to make changes to their files while they are on the Chrome browser. pdfFiller lets you make fillable documents and make changes to existing PDFs from any internet-connected device.
Can I create an electronic signature for signing my iso 13485 qms matrix in Gmail?
You can easily create your eSignature with pdfFiller and then eSign your iso 13485 qms matrix directly from your inbox with the help of pdfFiller’s add-on for Gmail. Please note that you must register for an account in order to save your signatures and signed documents.
How can I edit iso 13485 qms matrix on a smartphone?
Using pdfFiller's mobile-native applications for iOS and Android is the simplest method to edit documents on a mobile device. You may get them from the Apple App Store and Google Play, respectively. More information on the apps may be found here. Install the program and log in to begin editing iso 13485 qms matrix.
What is iso 13485 qms matrix?
The ISO 13485 QMS matrix is a document that outlines the quality management system requirements specified in the ISO 13485 standard.
Who is required to file iso 13485 qms matrix?
ISO 13485 QMS matrix is not filed but rather used by organizations that want to comply with the ISO 13485 standard for medical devices.
How to fill out iso 13485 qms matrix?
Filling out the ISO 13485 QMS matrix requires understanding the requirements of the ISO 13485 standard and mapping them to the organization's quality management system practices.
What is the purpose of iso 13485 qms matrix?
The purpose of the ISO 13485 QMS matrix is to provide a tool for organizations to assess and document their compliance with the ISO 13485 standard.
What information must be reported on iso 13485 qms matrix?
The ISO 13485 QMS matrix should include information on how the organization's quality management system addresses the requirements of the ISO 13485 standard.
Fill out your iso 13485 qms matrix online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Iso 13485 Qms Matrix is not the form you're looking for?Search for another form here.
Relevant keywords
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.














