Get the free ph and poh calculations worksheet
Show details
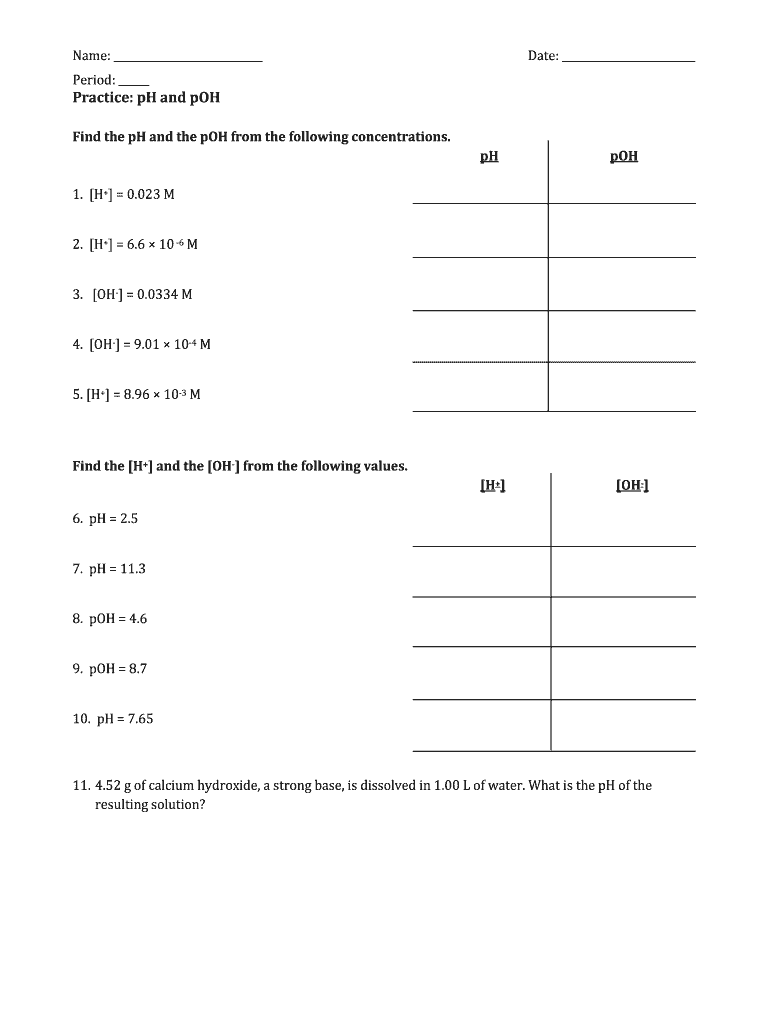
Name: Date: Period: Practice: pH and POH Find the pH and the POH from the following concentrations. 1. H+ 0.023 M pH POH 2. H+ 6.6 10 6 M 3. OH, 0.0334 M 4. OH, 9.01 104 M 5. H+ 8.96 103 M Find the
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign ph and poh worksheet form

Edit your ph calculations worksheet form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your ph and poh calculations worksheet with answers form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing calculating ph and poh worksheet online
Follow the guidelines below to benefit from the PDF editor's expertise:
1
Log in to your account. Start Free Trial and sign up a profile if you don't have one.
2
Upload a document. Select Add New on your Dashboard and transfer a file into the system in one of the following ways: by uploading it from your device or importing from the cloud, web, or internal mail. Then, click Start editing.
3
Edit ph and poh calculations worksheet pdf form. Add and change text, add new objects, move pages, add watermarks and page numbers, and more. Then click Done when you're done editing and go to the Documents tab to merge or split the file. If you want to lock or unlock the file, click the lock or unlock button.
4
Save your file. Select it from your records list. Then, click the right toolbar and select one of the various exporting options: save in numerous formats, download as PDF, email, or cloud.
With pdfFiller, it's always easy to work with documents. Try it out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out ph and poh calculations worksheet 2 form

How to fill out ph and poh calculations:
01
Determine the concentration of hydronium ions (H3O+) or hydroxide ions (OH-) in the solution.
02
Use the equation pH = -log[H3O+] to calculate the pH of the solution.
03
Use the equation pOH = -log[OH-] to calculate the pOH of the solution.
04
Calculate the pH and pOH if given the concentration of H3O+ or OH- ions.
Who needs ph and poh calculations:
01
Chemists and scientists who study acid-base reactions often need pH and pOH calculations for their research.
02
Individuals working in environmental science and water treatment industries use these calculations to determine the acidity or alkalinity of water samples.
03
Pharmacists and medical professionals may need pH and pOH calculations to understand the acidity or basicity of certain medications or physiological fluids.
Fill
how to get oh from ph
: Try Risk Free






People Also Ask about
What is the pH of 12 pOH?
Answer and Explanation: The correct answer is (b) 2. The pH scale is used to measure how acidic or basic a solution is.
How do you calculate pH and pOH?
11:46 13:49 And h3o plus x OE h minus is equal to 1 times 10 to the negative 14. Now in a similar way PKA isMoreAnd h3o plus x OE h minus is equal to 1 times 10 to the negative 14. Now in a similar way PKA is negative log of ka.
What is the pOH if the pH is 5?
When an aqueous solution has a pH of 5, it has a pOH of 9 (pH + pOH = 14). The formula for pOH is similar to that of pH, but uses the concentration of hydroxide ions instead of hydrogen ions.
What is the pOH if the pH is 3?
So, if the pH of your acidic solution is three, you can plug this in the equation above to find the pOH: Thus, For a 0.001 M solution of HCl, the pH is three, and the pOH is 11.
How do you convert pH to pOH?
0:03 5:26 Converting Between pH and pOH - YouTube YouTube Start of suggested clip End of suggested clip So pH equals 14 minus Poh. Or if you want to find Poh. Looking back up here. It's just Poh is equalMoreSo pH equals 14 minus Poh. Or if you want to find Poh. Looking back up here. It's just Poh is equal to 14 minus P subtract pH out from both sides. 14 minus 2h.
What is the pOH if the pH is 4?
Consider a solution with a pH =4.0. The [H+] of the solution would be 1.0×10−4M. Dividing Kw by this yields a [OH−] of 1.0×10−10M. Finally the pOH of the solution equals −log(1.0×10−10)=10.
What is the pOH of a 0.0235 M HCI solution?
pH = -log[H+] = -log(0.0235) = 1.629 2) What is the pOH of a 0.0235 M HCl solution?
How do you find pH and pOH without a calculator?
13:07 21:08 11 minus 0.1 that is 10.9. And 10.9 or 10.90 minus 0.03 90 minus 3 is 87. So this is going to beMore11 minus 0.1 that is 10.9. And 10.9 or 10.90 minus 0.03 90 minus 3 is 87. So this is going to be 10.87. So that's how we can estimate the poh of i mean the ph of this solution without a calculator.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
Can I create an electronic signature for signing my ph and poh calculations in Gmail?
When you use pdfFiller's add-on for Gmail, you can add or type a signature. You can also draw a signature. pdfFiller lets you eSign your ph and poh calculations and other documents right from your email. In order to keep signed documents and your own signatures, you need to sign up for an account.
How do I edit ph and poh calculations on an iOS device?
Use the pdfFiller app for iOS to make, edit, and share ph and poh calculations from your phone. Apple's store will have it up and running in no time. It's possible to get a free trial and choose a subscription plan that fits your needs.
How do I complete ph and poh calculations on an iOS device?
Install the pdfFiller app on your iOS device to fill out papers. Create an account or log in if you already have one. After registering, upload your ph and poh calculations. You may now use pdfFiller's advanced features like adding fillable fields and eSigning documents from any device, anywhere.
What is ph and poh calculations?
pH and pOH calculations are used to determine the acidity and basicity of a solution. The pH scale measures the concentration of hydrogen ions (H+) in a solution, while pOH measures the concentration of hydroxide ions (OH-). They are related by the equation pH + pOH = 14 at 25°C.
Who is required to file ph and poh calculations?
Individuals or entities involved in activities that generate wastewater or are regulated under environmental laws may be required to file pH and pOH calculations. This can include industries such as manufacturing, wastewater treatment, and research institutions.
How to fill out ph and poh calculations?
To fill out pH and pOH calculations, first measure the concentration of H+ ions for pH or OH- ions for pOH using appropriate methods (like a pH meter or titration). Then, calculate pH using the formula pH = -log[H+] and pOH using pOH = -log[OH-]. Ensure that the results are reported accurately according to the guidelines provided by regulatory agencies.
What is the purpose of ph and poh calculations?
The purpose of pH and pOH calculations is to assess the acidity or basicity of a solution, which is critical for environmental compliance, chemical processes, biological assessments, and ensuring safety in various applications.
What information must be reported on ph and poh calculations?
The information that must be reported in pH and pOH calculations typically includes the measured pH and pOH values, the concentration of H+ and OH- ions, the temperature of the solution during measurement, and any relevant methods used in obtaining the measurements.
Fill out your ph and poh calculations online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Ph And Poh Calculations is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.