Get the free INSTITUTIONAL BIOSAFETY REPORT FORM
Show details
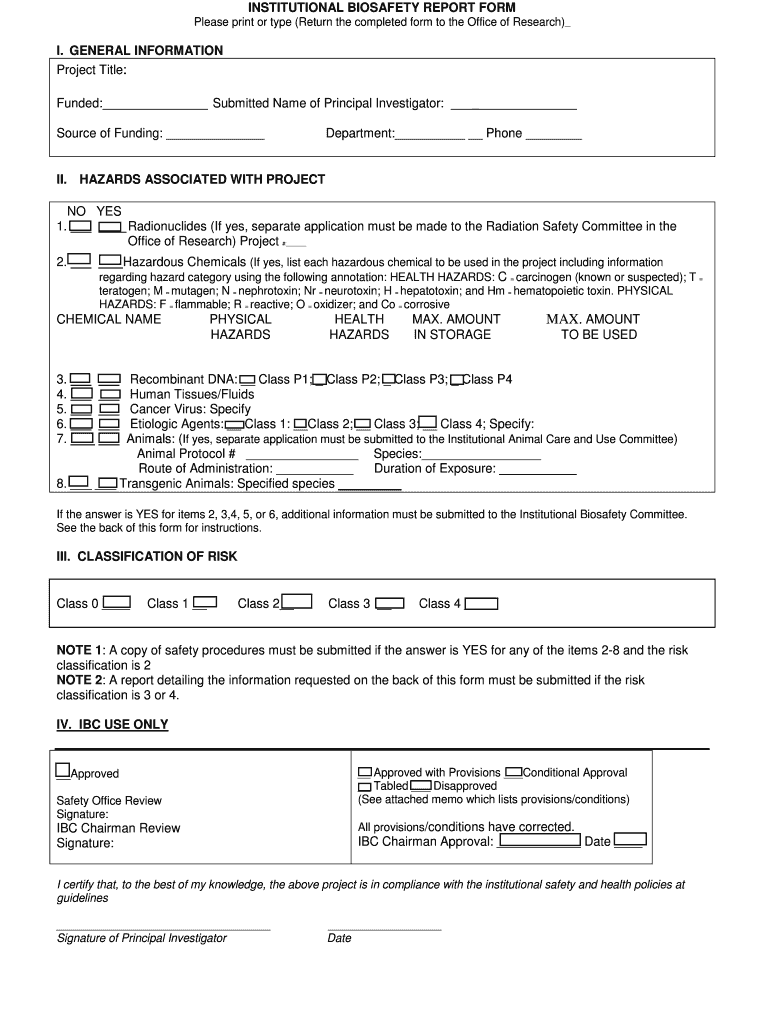
This form is used to report biosafety information related to research projects involving potential biohazards, hazardous chemicals, and other safety considerations, ensuring compliance with institutional
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign institutional biosafety report form

Edit your institutional biosafety report form form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your institutional biosafety report form form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit institutional biosafety report form online
Follow the guidelines below to take advantage of the professional PDF editor:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit institutional biosafety report form. Text may be added and replaced, new objects can be included, pages can be rearranged, watermarks and page numbers can be added, and so on. When you're done editing, click Done and then go to the Documents tab to combine, divide, lock, or unlock the file.
4
Get your file. When you find your file in the docs list, click on its name and choose how you want to save it. To get the PDF, you can save it, send an email with it, or move it to the cloud.
With pdfFiller, it's always easy to deal with documents.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out institutional biosafety report form

How to fill out INSTITUTIONAL BIOSAFETY REPORT FORM
01
Obtain the INSTITUTIONAL BIOSAFETY REPORT FORM from your institution's biosafety office.
02
Fill out the section for institutional information, including the institution's name and address.
03
Provide the principal investigator's (PI) details, including name, contact information, and department.
04
Describe the nature of the research or activity that requires biosafety consideration.
05
Indicate the type of biological materials involved, including any pathogens or genetically modified organisms.
06
Assess and outline the potential biosafety risks associated with the research.
07
Provide details on safety measures and containment procedures that will be implemented.
08
Review the form thoroughly for accuracy and completeness.
09
Sign and date the form where required.
10
Submit the completed INSTITUTIONAL BIOSAFETY REPORT FORM to the appropriate biosafety committee for review.
Who needs INSTITUTIONAL BIOSAFETY REPORT FORM?
01
Researchers and principal investigators conducting activities involving biological materials.
02
Laboratory personnel working with potentially hazardous biological agents.
03
Institutions that are engaged in research activities requiring biosafety compliance and oversight.
Fill
form
: Try Risk Free






People Also Ask about
What should an institutional biosafety committee (IBC) include in the annual report to the NIH OSP?
The Institutional Biosafety Committee (IBC) application is an online questionnaire/form that you complete in the eResearch Regulatory Management (eRRM) system to describe all of your work with potentially hazardous biologics, including recombinant DNA and synthetic nucleic acid molecules.
Is IBC required?
IBC approval is required when research involves human source material, such as blood and body tissues, and when biohazardous material is used in animal research, among other cases.
What is IBC registration?
The IBC provides recommendations to the intramural community in matters pertaining to the control of biohazards associated with the use of microbiological agents and their vectors. It also represents the interests of the surrounding community with respect to public health and protection of the environment.
What is an IBC registration?
The Institutional Biosafety Committee (IBC) application is an online questionnaire/form that you complete in the eResearch Regulatory Management (eRRM) system to describe all of your work with potentially hazardous biologics, including recombinant DNA and synthetic nucleic acid molecules.
Who needs IBC approval?
Institutional Biosafety Committee (IBC) approval is required before conducting research and teaching activities involving rDNA, biohazardous agents, materials, and toxins.
What is an institutional biosafety committee?
Some examples of human subject research requiring IBC oversight include the following, among other activities: Serial blood sampling or the processing of human blood during alcohol consumption or smoking studies. Utilizing recombinant DNA to study how viruses attach and move within human cells.
What is the purpose of an IBC?
Institutional Biosafety Committees (IBCs) were established under the NIH Guidelines to provide local review and oversight of nearly all forms of research utilizing recombinant or synthetic nucleic acid molecules.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is INSTITUTIONAL BIOSAFETY REPORT FORM?
The INSTITUTIONAL BIOSAFETY REPORT FORM is a document required for reporting and assessing potential biosafety risks associated with research and activities involving biohazardous materials.
Who is required to file INSTITUTIONAL BIOSAFETY REPORT FORM?
Researchers, institutions, or organizations conducting studies involving biohazardous materials, including genetically modified organisms, are required to file this form.
How to fill out INSTITUTIONAL BIOSAFETY REPORT FORM?
To fill out the INSTITUTIONAL BIOSAFETY REPORT FORM, provide detailed information about the research project, including the type of biological materials used, the purpose of the research, and safety measures implemented.
What is the purpose of INSTITUTIONAL BIOSAFETY REPORT FORM?
The purpose of the INSTITUTIONAL BIOSAFETY REPORT FORM is to ensure the safe handling and containment of biohazardous materials, to protect public health and the environment, and to comply with regulatory requirements.
What information must be reported on INSTITUTIONAL BIOSAFETY REPORT FORM?
The report must include information such as the researcher’s details, project description, types of organisms used, potential biosafety risks, intended use of the materials, and containment measures in place.
Fill out your institutional biosafety report form online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Institutional Biosafety Report Form is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















