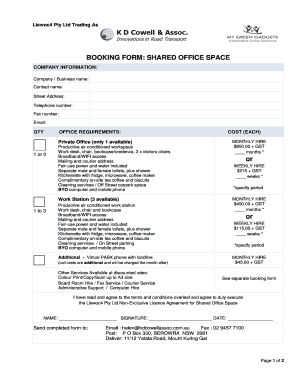
Get the free Center for Clinical Research Solutions Inc Request for
Show details
Center for Clinical Research Solutions, Inc. Request for Proposal Please select the services you require and provide as much detail as possible. You may email or fax this form to Jennifer Hawaii,
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign center for clinical research

Edit your center for clinical research form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your center for clinical research form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit center for clinical research online
In order to make advantage of the professional PDF editor, follow these steps:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit center for clinical research. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Save your file. Select it from your list of records. Then, move your cursor to the right toolbar and choose one of the exporting options. You can save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud, among other things.
Dealing with documents is always simple with pdfFiller.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out center for clinical research

How to fill out a center for clinical research:
01
Identify the purpose: Before filling out a center for clinical research, it is important to clearly define the purpose of the center. This can include conducting clinical trials, collecting and analyzing data, collaborating with researchers, or providing resources for clinical research.
02
Determine the location: Choose a suitable location for the center that is accessible to healthcare facilities, research institutions, and participants. Consider factors such as proximity to medical schools, hospitals, and patient populations.
03
Establish infrastructure: Set up the necessary infrastructure for the center, including office space, laboratories, equipment, and technology required for conducting research. This may involve coordinating with architects, engineers, and IT professionals to ensure the facility meets the research requirements.
04
Recruit qualified staff: Assemble a team of skilled professionals who can contribute to the success of the center. This can include researchers, clinicians, nurses, data analysts, project managers, and administrative staff. Ensure that each team member has the necessary qualifications and experience in clinical research.
05
Obtain necessary approvals: Before commencing any research activities, it is vital to obtain the required approvals and certifications. This may include obtaining ethical clearance from an institutional review board, registering the center with regulatory bodies, and complying with any local, state, or national regulations governing clinical research.
06
Develop protocols and procedures: Create comprehensive protocols and procedures that outline the steps involved in conducting clinical research at the center. This includes documenting guidelines for participant recruitment, data collection, informed consent, study conduct, adverse event reporting, and data management.
07
Establish collaborations: Establish collaborations and partnerships with research institutions, pharmaceutical companies, healthcare providers, and other stakeholders to enhance the research capabilities of the center. This can involve seeking funding opportunities, sharing resources, and gaining access to a diverse range of research studies.
08
Train and educate staff: Provide ongoing training and education to the staff members to ensure they are up-to-date with the latest research methodologies, regulatory requirements, and ethical standards. This can include organizing workshops, seminars, and online courses, as well as encouraging staff to pursue professional certifications in clinical research.
09
Implement quality assurance measures: Implement quality assurance measures to ensure that the research conducted at the center meets the highest standards of accuracy, integrity, and ethical considerations. This may involve regular audits, inspections, and internal reviews to identify and address any deviations or non-compliance.
10
Continuously improve: Foster a culture of continuous improvement by regularly assessing the performance of the center and implementing necessary changes. This can include conducting evaluations, collecting feedback from researchers and participants, and staying updated with advancements in clinical research methodologies and technologies.
Who needs a center for clinical research?
01
Pharmaceutical companies: Pharmaceutical companies require centers for clinical research to conduct trials and gather data on the effectiveness and safety of their drugs or medical devices before seeking regulatory approval.
02
Medical researchers: Medical researchers rely on centers for clinical research to conduct studies that aim to improve medical knowledge and develop new treatments, interventions, or diagnostic tools.
03
Healthcare providers: Centers for clinical research provide healthcare providers with opportunities to collaborate on research studies, gain access to innovative treatments or techniques, and contribute to the advancement of medical science.
04
Patients and participants: Patients and participants benefit from centers for clinical research as they may have access to cutting-edge treatments or therapies that are not yet available to the general public. Additionally, participating in research studies can provide patients with a sense of contributing to medical advancements and potentially improving their own health outcomes.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is center for clinical research?
Center for clinical research is a specialized facility where clinical trials and research studies are conducted.
Who is required to file center for clinical research?
Research institutions, universities, pharmaceutical companies, and other organizations involved in clinical research are required to file center for clinical research.
How to fill out center for clinical research?
Center for clinical research can be filled out online or submitted through the appropriate regulatory authorities.
What is the purpose of center for clinical research?
The purpose of center for clinical research is to track and monitor the progress of clinical trials, ensure compliance with regulations, and analyze study data.
What information must be reported on center for clinical research?
Information such as study protocols, informed consent forms, adverse event reports, and study results must be reported on center for clinical research.
How do I fill out the center for clinical research form on my smartphone?
The pdfFiller mobile app makes it simple to design and fill out legal paperwork. Complete and sign center for clinical research and other papers using the app. Visit pdfFiller's website to learn more about the PDF editor's features.
How can I fill out center for clinical research on an iOS device?
Install the pdfFiller app on your iOS device to fill out papers. Create an account or log in if you already have one. After registering, upload your center for clinical research. You may now use pdfFiller's advanced features like adding fillable fields and eSigning documents from any device, anywhere.
How do I complete center for clinical research on an Android device?
Complete center for clinical research and other documents on your Android device with the pdfFiller app. The software allows you to modify information, eSign, annotate, and share files. You may view your papers from anywhere with an internet connection.
Fill out your center for clinical research online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Center For Clinical Research is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.