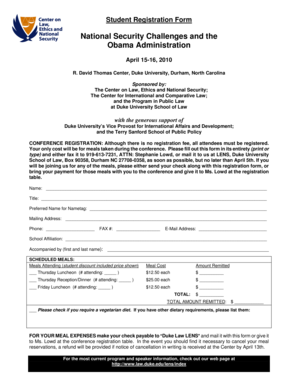
Get the free Guidelines for Laboratory Verification of Performance of the FilmArray RP System ADV...
Show details
Guidelines for Laboratory Verification of Performance of the Disarray RP System ADVISORY ::: NOTICE Purpose The Clinical Laboratory Improvement Amendments (CIA), passed in 1988, establishes quality
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign guidelines for laboratory verification
Edit your guidelines for laboratory verification form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.
Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your guidelines for laboratory verification form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit guidelines for laboratory verification online
To use our professional PDF editor, follow these steps:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Upload a document. Select Add New on your Dashboard and transfer a file into the system in one of the following ways: by uploading it from your device or importing from the cloud, web, or internal mail. Then, click Start editing.
3
Edit guidelines for laboratory verification. Add and change text, add new objects, move pages, add watermarks and page numbers, and more. Then click Done when you're done editing and go to the Documents tab to merge or split the file. If you want to lock or unlock the file, click the lock or unlock button.
4
Save your file. Choose it from the list of records. Then, shift the pointer to the right toolbar and select one of the several exporting methods: save it in multiple formats, download it as a PDF, email it, or save it to the cloud.
The use of pdfFiller makes dealing with documents straightforward.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out guidelines for laboratory verification
How to fill out guidelines for laboratory verification:
01
Start by reviewing any existing guidelines or templates provided by the regulatory agency or organization overseeing the laboratory verification process.
02
Familiarize yourself with the specific requirements and criteria that need to be addressed in the guidelines. This may include information on laboratory standards, equipment validation, quality control measures, and data management, among other things.
03
Begin by providing a clear and concise introduction to the guidelines, explaining their purpose and scope. This will set the context for the rest of the document.
04
Structure the guidelines into sections or categories based on the different aspects of laboratory verification that need to be covered. This can help ensure that all relevant information is included and organized in a logical manner.
05
For each section, provide detailed instructions and guidance on how to meet the specific requirements or criteria. This may involve outlining the necessary steps, procedures, and documentation that need to be followed.
06
Include any relevant forms, templates, or checklists that laboratories may need to use when conducting the verification process. These can help streamline the process and ensure consistency.
07
Clearly define any key terms or terminology used throughout the guidelines to avoid any confusion or misunderstanding.
08
Consider including examples or case studies to illustrate how the guidelines can be applied in different laboratory settings. This can help laboratories better understand how to implement the verification process effectively.
09
Once the guidelines are complete, review them for clarity, consistency, and accuracy. Make sure all relevant information is included and that the guidelines align with the regulatory requirements.
10
Finally, distribute the guidelines to the relevant stakeholders, such as laboratory managers, personnel, and quality control teams, who need to follow and adhere to them for laboratory verification.
Who needs guidelines for laboratory verification?
01
Laboratories seeking to comply with regulatory requirements and standards for laboratory verification.
02
Laboratory managers and personnel responsible for overseeing the verification process and ensuring compliance.
03
Quality control teams and auditors who need to evaluate and assess the laboratory's verification procedures and practices.
04
Regulatory agencies or organizations that set the guidelines and standards for laboratory verification and require laboratories to adhere to them.
05
Researchers or scientists conducting laboratory experiments or studies that require accurate and reliable verification of their results.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I send guidelines for laboratory verification for eSignature?
guidelines for laboratory verification is ready when you're ready to send it out. With pdfFiller, you can send it out securely and get signatures in just a few clicks. PDFs can be sent to you by email, text message, fax, USPS mail, or notarized on your account. You can do this right from your account. Become a member right now and try it out for yourself!
How do I edit guidelines for laboratory verification in Chrome?
Get and add pdfFiller Google Chrome Extension to your browser to edit, fill out and eSign your guidelines for laboratory verification, which you can open in the editor directly from a Google search page in just one click. Execute your fillable documents from any internet-connected device without leaving Chrome.
Can I sign the guidelines for laboratory verification electronically in Chrome?
Yes. By adding the solution to your Chrome browser, you can use pdfFiller to eSign documents and enjoy all of the features of the PDF editor in one place. Use the extension to create a legally-binding eSignature by drawing it, typing it, or uploading a picture of your handwritten signature. Whatever you choose, you will be able to eSign your guidelines for laboratory verification in seconds.
What is guidelines for laboratory verification?
The guidelines for laboratory verification outline the procedures and requirements for ensuring the accuracy and reliability of laboratory test results.
Who is required to file guidelines for laboratory verification?
Laboratories and testing facilities are required to file guidelines for laboratory verification.
How to fill out guidelines for laboratory verification?
Guidelines for laboratory verification can be filled out by providing accurate information about the laboratory's procedures and protocols.
What is the purpose of guidelines for laboratory verification?
The purpose of guidelines for laboratory verification is to ensure the quality and accuracy of laboratory test results.
What information must be reported on guidelines for laboratory verification?
Information such as the laboratory's quality control measures, equipment calibration procedures, and personnel training programs must be reported on guidelines for laboratory verification.
Fill out your guidelines for laboratory verification online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Guidelines For Laboratory Verification is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.