
Get the free Clinical Data Sheet - Arkana Laboratories
Show details
Clinical Data Sheet Ordering Physician: Patient Name: Relevant History and Data: Other: Appropriate Clinical Syndrome: Labs: Sexologies: Time Frame: Age: Race:
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign clinical data sheet
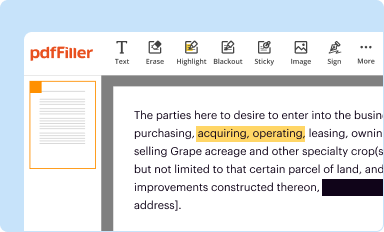
Edit your clinical data sheet form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.
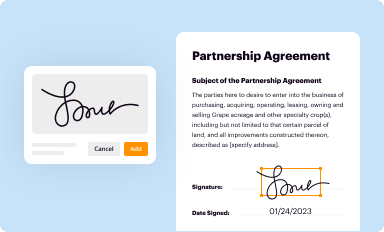
Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your clinical data sheet form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit clinical data sheet online
To use our professional PDF editor, follow these steps:
1
Log in to account. Start Free Trial and register a profile if you don't have one.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit clinical data sheet. Add and replace text, insert new objects, rearrange pages, add watermarks and page numbers, and more. Click Done when you are finished editing and go to the Documents tab to merge, split, lock or unlock the file.
4
Save your file. Select it from your records list. Then, click the right toolbar and select one of the various exporting options: save in numerous formats, download as PDF, email, or cloud.
pdfFiller makes dealing with documents a breeze. Create an account to find out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out clinical data sheet
How to fill out a clinical data sheet:
01
Start by gathering all the necessary information. This may include the patient's personal details such as name, age, gender, contact information, and any relevant identification numbers.
02
Next, document the patient's medical history, including any existing conditions, past surgeries or procedures, and current medications being taken. It is important to be thorough and include any pertinent details that may impact the patient's treatment.
03
Record the vital signs and relevant measurements such as blood pressure, heart rate, temperature, and height/weight.
04
Document any symptoms or complaints that the patient is experiencing, as well as the date and time they were first noticed.
05
Include information about any current or recent treatments, therapies, or medications that the patient is undergoing.
06
If applicable, note any allergies or sensitivities that the patient may have.
07
Include any relevant laboratory or diagnostic test results, such as blood tests, X-rays, or MRIs. Be sure to specify the date the tests were conducted and the name of the healthcare provider who ordered them.
08
Lastly, provide a space for any additional notes or comments that may be relevant to the patient's condition or treatment.
Who needs a clinical data sheet:
01
Healthcare professionals, such as doctors, nurses, and medical researchers, require clinical data sheets to effectively assess and monitor a patient's health status and provide appropriate care.
02
Clinical trial investigators and research personnel need clinical data sheets to collect comprehensive information about participants' medical history, treatment outcomes, and any adverse events encountered during the study.
03
Insurance companies may also utilize clinical data sheets to evaluate claims and determine coverage eligibility based on the provided medical information.
04
In some cases, patients may also benefit from having their own copy of the clinical data sheet to keep track of their medical history, appointments, and treatment plans.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I modify my clinical data sheet in Gmail?
You may use pdfFiller's Gmail add-on to change, fill out, and eSign your clinical data sheet as well as other documents directly in your inbox by using the pdfFiller add-on for Gmail. pdfFiller for Gmail may be found on the Google Workspace Marketplace. Use the time you would have spent dealing with your papers and eSignatures for more vital tasks instead.
How do I make changes in clinical data sheet?
pdfFiller not only allows you to edit the content of your files but fully rearrange them by changing the number and sequence of pages. Upload your clinical data sheet to the editor and make any required adjustments in a couple of clicks. The editor enables you to blackout, type, and erase text in PDFs, add images, sticky notes and text boxes, and much more.
Can I create an electronic signature for signing my clinical data sheet in Gmail?
With pdfFiller's add-on, you may upload, type, or draw a signature in Gmail. You can eSign your clinical data sheet and other papers directly in your mailbox with pdfFiller. To preserve signed papers and your personal signatures, create an account.
What is clinical data sheet?
Clinical data sheet is a document that contains detailed information about the clinical trial, including study protocol, patient demographics, treatment regimen, adverse events, and study outcomes.
Who is required to file clinical data sheet?
The sponsor or organization conducting the clinical trial is required to file the clinical data sheet.
How to fill out clinical data sheet?
The clinical data sheet can be filled out electronically or manually, following the specific instructions provided by the regulatory authorities or ethics committees.
What is the purpose of clinical data sheet?
The purpose of the clinical data sheet is to provide a comprehensive overview of the clinical trial, ensuring transparency, accountability, and data integrity.
What information must be reported on clinical data sheet?
The clinical data sheet must include information on study design, enrollment criteria, treatment plan, adverse events, efficacy outcomes, and patient safety measures.
Fill out your clinical data sheet online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Clinical Data Sheet is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















