Get the free Lentivirus Production Report - ors.ubc.ca - ors ubc
Show details
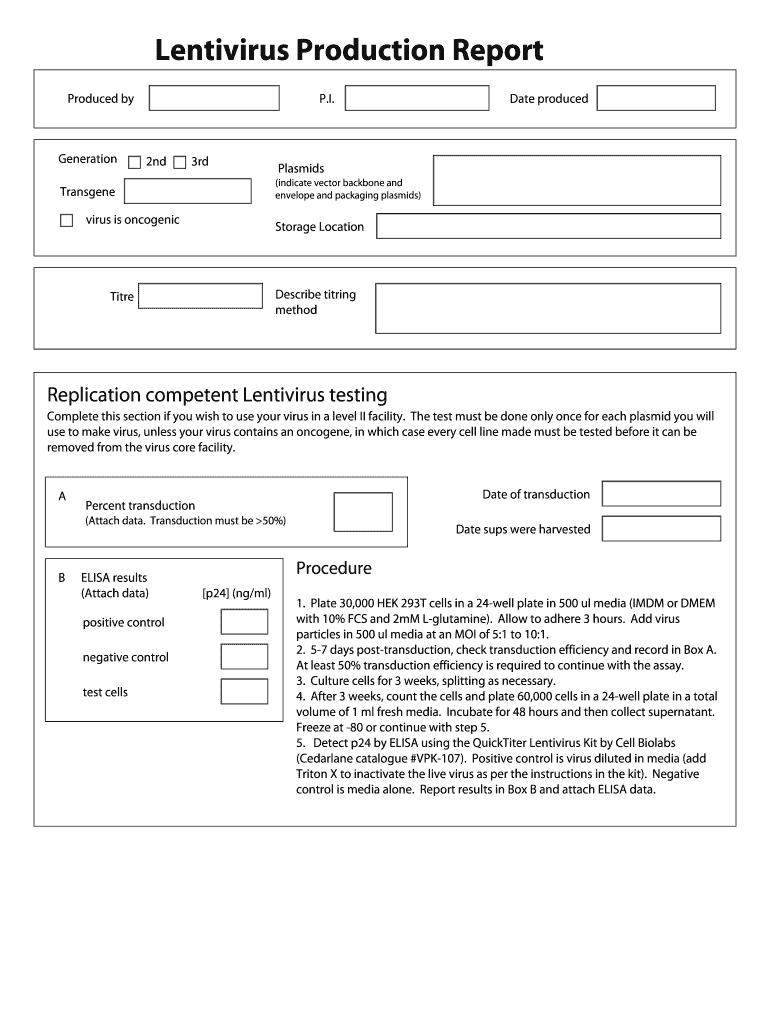
Antivirus Production Report. Produced by Plasmids (indicate vector backbone and. Transgene envelope and packaging plasmids) Date produced Describe tiring.
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign lentivirus production report

Edit your lentivirus production report form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your lentivirus production report form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing lentivirus production report online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Log in. Click Start Free Trial and create a profile if necessary.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit lentivirus production report. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Save your file. Select it from your list of records. Then, move your cursor to the right toolbar and choose one of the exporting options. You can save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud, among other things.
With pdfFiller, it's always easy to deal with documents. Try it right now
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out lentivirus production report

How to fill out a lentivirus production report:
01
Start by providing the necessary identification information, such as the date of production, the name of the person or laboratory responsible for the production, and any relevant project or experiment numbers.
02
Specify the lentivirus vector used in the production, including its name, source, and any modifications or modifications made to it.
03
Outline the production process, detailing the steps involved, such as cell culture preparation, transfection or infection protocols, and any selection or enrichment methods used.
04
Record the relevant parameters and measurements during the production, such as cell density, virus titer, transduction efficiency, and any other quality control tests conducted.
05
Include details about any additives or supplements used during the production, such as growth factors, cytokines, or antibiotics.
06
Provide information about the equipment and materials used, including the type of cell culture vessels, media, reagents, and equipment, such as bioreactors or centrifuges.
07
Note down any deviations or variations from the standard production protocol, along with the reasons and any corrective actions taken.
08
Include any additional comments or observations that may be relevant for future reference or troubleshooting purposes.
Who needs a lentivirus production report?
Lentivirus production reports are typically needed by researchers, scientists, and laboratories involved in gene therapy, gene editing, or other molecular biology applications. These reports serve as documentation of the lentivirus production process, allowing for better reproducibility, data analysis, and quality control. Additionally, the lentivirus production reports may be required for regulatory compliance or when sharing or publishing research findings with the scientific community.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I edit lentivirus production report from Google Drive?
By integrating pdfFiller with Google Docs, you can streamline your document workflows and produce fillable forms that can be stored directly in Google Drive. Using the connection, you will be able to create, change, and eSign documents, including lentivirus production report, all without having to leave Google Drive. Add pdfFiller's features to Google Drive and you'll be able to handle your documents more effectively from any device with an internet connection.
How can I fill out lentivirus production report on an iOS device?
Install the pdfFiller iOS app. Log in or create an account to access the solution's editing features. Open your lentivirus production report by uploading it from your device or online storage. After filling in all relevant fields and eSigning if required, you may save or distribute the document.
How do I edit lentivirus production report on an Android device?
With the pdfFiller mobile app for Android, you may make modifications to PDF files such as lentivirus production report. Documents may be edited, signed, and sent directly from your mobile device. Install the app and you'll be able to manage your documents from anywhere.
What is lentivirus production report?
The lentivirus production report is a document that details the production of lentivirus vectors.
Who is required to file lentivirus production report?
Any organization or individual involved in the production of lentivirus vectors is required to file the lentivirus production report.
How to fill out lentivirus production report?
The lentivirus production report can be filled out by providing detailed information about the production process, including the quantity of lentivirus vectors produced and any relevant quality control measures.
What is the purpose of lentivirus production report?
The purpose of the lentivirus production report is to ensure transparency and accountability in the production of lentivirus vectors.
What information must be reported on lentivirus production report?
Information such as the quantity of lentivirus vectors produced, production methods, quality control measures, and any deviations from standard procedures must be reported on the lentivirus production report.
Fill out your lentivirus production report online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Lentivirus Production Report is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.




















