Get the free Prospective, Randomized, Multi-Center Trial of Lateral ... - compartint
Show details
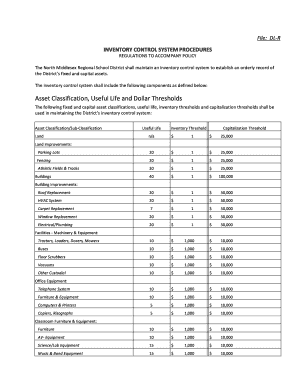
Prospective, Randomized, Multimeter Trial of Lateral Trendelenburg Versus SemiRecumbent Body Position in Mechanically Ventilated Patients For The Prevention of VentilatorAssociated Pneumonia Version
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign prospective randomized multi-center trial

Edit your prospective randomized multi-center trial form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your prospective randomized multi-center trial form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing prospective randomized multi-center trial online
Use the instructions below to start using our professional PDF editor:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit prospective randomized multi-center trial. Rearrange and rotate pages, insert new and alter existing texts, add new objects, and take advantage of other helpful tools. Click Done to apply changes and return to your Dashboard. Go to the Documents tab to access merging, splitting, locking, or unlocking functions.
4
Save your file. Select it from your records list. Then, click the right toolbar and select one of the various exporting options: save in numerous formats, download as PDF, email, or cloud.
It's easier to work with documents with pdfFiller than you could have believed. You may try it out for yourself by signing up for an account.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out prospective randomized multi-center trial

How to fill out a prospective randomized multi-center trial:
01
Clearly define the research question: Before filling out the trial, it is essential to have a clear understanding of the research question that the trial aims to answer. This ensures that the trial design and data collection methods are aligned with the research objectives.
02
Plan the study design: Decide on the appropriate study design for your prospective randomized multi-center trial. This includes determining the number of study centers, randomization procedures, allocation ratio, and sample size calculation. Consider the practicality and feasibility of the study design.
03
Obtain necessary approvals: Ensure that the trial protocol is reviewed and approved by the relevant ethics committees and regulatory authorities. This step is crucial to protect the rights and welfare of the trial participants and ensure compliance with ethical standards.
04
Recruit study centers and collaborators: Identify suitable study centers and collaborate with researchers or clinicians who are willing to participate in the trial. Establish clear communication channels and provide them with all the necessary resources and training needed to conduct the trial effectively.
05
Develop standardized data collection tools: Design and develop standardized data collection tools such as case report forms (CRFs) or electronic data capture (EDC) systems. These tools should capture all the required data points as per the study protocol, ensuring consistency and accuracy in data collection across the multiple centers.
06
Conduct training and site initiation visits: Conduct comprehensive training sessions for the investigators and research staff involved in the trial. This includes training on study procedures, data management, adverse event reporting, regulatory compliance, and any specific interventions or treatments being investigated. Site initiation visits can also be conducted to ensure that all study centers are adequately prepared to enroll and manage participants.
07
Implement the trial and monitor progress: Once the trial is initiated, ongoing monitoring activities are essential to ensure compliance with the protocol, identify and address any issues or challenges faced by the study centers, and ensure the quality and integrity of the data being collected. This may involve regular site visits, remote monitoring, or data audits.
08
Analyze and interpret the data: Once the trial is completed, the collected data needs to be analyzed using appropriate statistical methods. The results should be interpreted in the context of the research question and presented in a clear and meaningful manner.
09
Disseminate the findings: The results of the prospective randomized multi-center trial should be disseminated through peer-reviewed publications and conferences to contribute to the scientific knowledge and inform clinical practice. It is essential to communicate the findings to relevant stakeholders, including healthcare professionals, policymakers, and patient groups.
Who needs prospective randomized multi-center trial?
Prospective randomized multi-center trials are often needed in clinical research and healthcare settings where there is a need to evaluate the safety and efficacy of new interventions or treatments across multiple centers. They are commonly conducted in areas such as pharmaceutical drug development, medical device testing, or comparative effectiveness research. Researchers, pharmaceutical companies, clinicians, regulatory authorities, and healthcare policymakers are among the key stakeholders who may benefit from the results of prospective randomized multi-center trials.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I manage my prospective randomized multi-center trial directly from Gmail?
You may use pdfFiller's Gmail add-on to change, fill out, and eSign your prospective randomized multi-center trial as well as other documents directly in your inbox by using the pdfFiller add-on for Gmail. pdfFiller for Gmail may be found on the Google Workspace Marketplace. Use the time you would have spent dealing with your papers and eSignatures for more vital tasks instead.
Can I create an electronic signature for the prospective randomized multi-center trial in Chrome?
Yes, you can. With pdfFiller, you not only get a feature-rich PDF editor and fillable form builder but a powerful e-signature solution that you can add directly to your Chrome browser. Using our extension, you can create your legally-binding eSignature by typing, drawing, or capturing a photo of your signature using your webcam. Choose whichever method you prefer and eSign your prospective randomized multi-center trial in minutes.
Can I edit prospective randomized multi-center trial on an iOS device?
Use the pdfFiller mobile app to create, edit, and share prospective randomized multi-center trial from your iOS device. Install it from the Apple Store in seconds. You can benefit from a free trial and choose a subscription that suits your needs.
What is prospective randomized multi-center trial?
A prospective randomized multi-center trial is a type of clinical study in which participants are assigned to different treatment groups randomly and the trial is conducted at multiple locations.
Who is required to file prospective randomized multi-center trial?
Researchers, scientists, or organizations conducting clinical trials are required to file prospective randomized multi-center trials.
How to fill out prospective randomized multi-center trial?
Prospective randomized multi-center trials must be filled out following the specific guidelines and protocols of the trial design, including information on study objectives, interventions, participant eligibility criteria, and outcome measures.
What is the purpose of prospective randomized multi-center trial?
The purpose of prospective randomized multi-center trials is to evaluate the effectiveness and safety of new treatments or interventions in a controlled and systematic way.
What information must be reported on prospective randomized multi-center trial?
Information such as study objectives, eligibility criteria, treatment arms, randomization procedures, participant characteristics, adverse events, and study outcomes must be reported on prospective randomized multi-center trials.
Fill out your prospective randomized multi-center trial online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Prospective Randomized Multi-Center Trial is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.