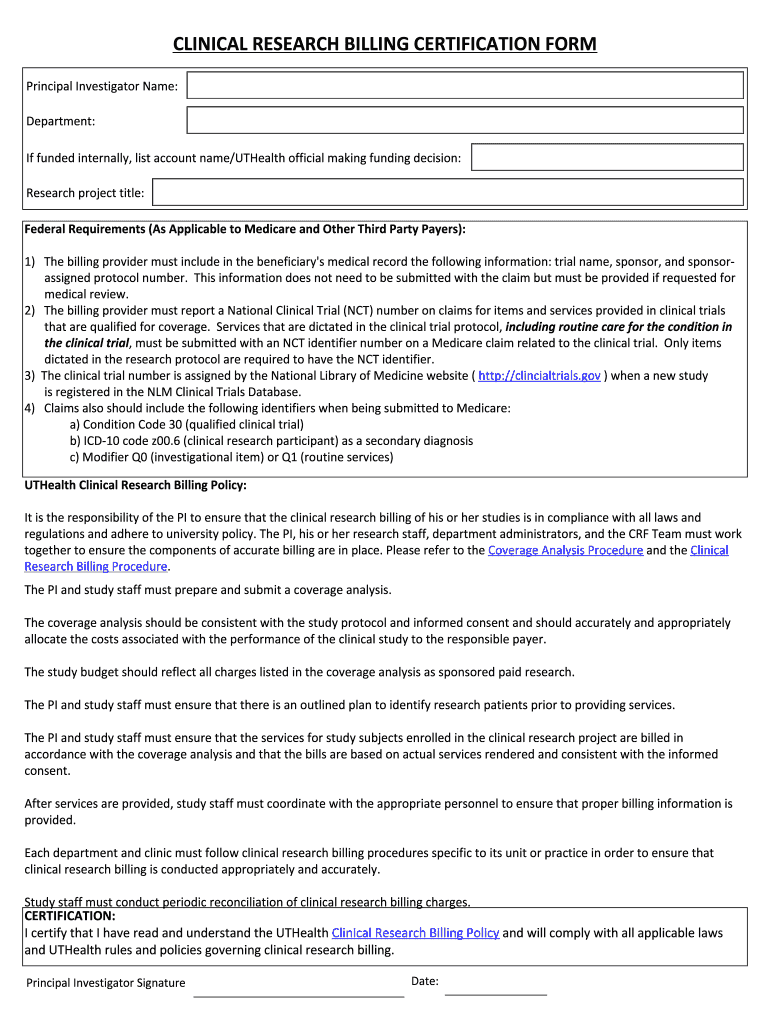
Get the free CLINICAL RESEARCH BILLING CERTIFICATION FORM
Show details
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign clinical research billing certification

Edit your clinical research billing certification form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your clinical research billing certification form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing clinical research billing certification online
To use the professional PDF editor, follow these steps:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Upload a document. Select Add New on your Dashboard and transfer a file into the system in one of the following ways: by uploading it from your device or importing from the cloud, web, or internal mail. Then, click Start editing.
3
Edit clinical research billing certification. Replace text, adding objects, rearranging pages, and more. Then select the Documents tab to combine, divide, lock or unlock the file.
4
Save your file. Select it from your records list. Then, click the right toolbar and select one of the various exporting options: save in numerous formats, download as PDF, email, or cloud.
It's easier to work with documents with pdfFiller than you can have believed. Sign up for a free account to view.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out clinical research billing certification

How to fill out clinical research billing certification?
01
Start by gathering all necessary documents and information. This may include your personal identification, academic qualifications, and previous clinical research billing experience.
02
Research the specific requirements and guidelines for clinical research billing certification in your country or region. Understand the necessary steps and any supporting documentation that may be required.
03
Complete the application form accurately and legibly. Pay attention to any specific instructions or sections that may require additional information or supporting documents.
04
Provide any requested supporting documentation, such as educational certificates or proof of previous clinical research billing experience. Make sure to submit copies of these documents and keep the originals for your records.
05
Double-check all the information provided on the application form before submitting it. Ensure that all fields are filled in correctly and any required signatures are included.
06
Pay the necessary fees, if applicable, for processing the certification application. Follow the payment instructions provided by the certification body.
07
Submit your completed application, supporting documents, and payment (if required) to the designated certification body. Send the application via mail or submit it online, following the specified instructions.
08
After submitting the application, wait for the certification body's response. This may involve a verification process or further evaluation of your application and supporting documents.
09
If your application is approved, you will receive your clinical research billing certification. If there are any missing or incorrect details, the certification body may request further information or clarification.
10
Upon receiving your certification, ensure that you maintain compliance with any ongoing requirements or continuing education necessary to retain your clinical research billing certification.
Who needs clinical research billing certification?
01
Individuals working in the field of clinical research who handle billing and financial aspects of research studies.
02
Professionals involved in academic or industry-sponsored clinical trials or studies.
03
Billing managers, coordinators, and administrators in research institutes, hospitals, or healthcare organizations.
04
Finance or accounting professionals responsible for managing the financial aspects of clinical research studies.
05
Individuals seeking to enhance their career prospects and demonstrate their competency in clinical research billing and financial management.
06
Those seeking to comply with regulatory requirements or industry standards that may mandate the need for clinical research billing certification.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I edit clinical research billing certification on a smartphone?
The easiest way to edit documents on a mobile device is using pdfFiller’s mobile-native apps for iOS and Android. You can download those from the Apple Store and Google Play, respectively. You can learn more about the apps here. Install and log in to the application to start editing clinical research billing certification.
How do I fill out clinical research billing certification using my mobile device?
You can quickly make and fill out legal forms with the help of the pdfFiller app on your phone. Complete and sign clinical research billing certification and other documents on your mobile device using the application. If you want to learn more about how the PDF editor works, go to pdfFiller.com.
Can I edit clinical research billing certification on an iOS device?
Use the pdfFiller mobile app to create, edit, and share clinical research billing certification from your iOS device. Install it from the Apple Store in seconds. You can benefit from a free trial and choose a subscription that suits your needs.
What is clinical research billing certification?
Clinical research billing certification is a process that involves ensuring accurate and compliant billing practices for clinical trial services.
Who is required to file clinical research billing certification?
Research institutions and organizations conducting clinical trials are required to file clinical research billing certification.
How to fill out clinical research billing certification?
Clinical research billing certification can typically be filled out online through a designated platform provided by regulatory authorities.
What is the purpose of clinical research billing certification?
The purpose of clinical research billing certification is to prevent billing errors, ensure compliance with regulations, and protect against fraud and abuse.
What information must be reported on clinical research billing certification?
Clinical research billing certification typically requires information on the clinical trial protocol, services provided, personnel involved, and payment details.
Fill out your clinical research billing certification online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Clinical Research Billing Certification is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.


















