Get the free Site Validation Master Plan
Show details
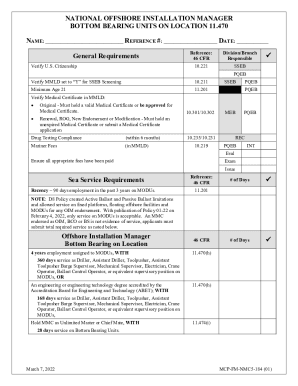
Site Validation Master PlanDocument Tracking# Rev#
Company Accompany Name Page 1 of 34Site Validation Master PlanDocument Tracking# Rev#
Company Allocation: Location Site Validation Master PlanDOCUMENT
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign site validation master plan

Edit your site validation master plan form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your site validation master plan form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing site validation master plan online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit site validation master plan. Add and change text, add new objects, move pages, add watermarks and page numbers, and more. Then click Done when you're done editing and go to the Documents tab to merge or split the file. If you want to lock or unlock the file, click the lock or unlock button.
4
Save your file. Select it from your records list. Then, click the right toolbar and select one of the various exporting options: save in numerous formats, download as PDF, email, or cloud.
It's easier to work with documents with pdfFiller than you can have ever thought. Sign up for a free account to view.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out site validation master plan

How to fill out site validation master plan:
01
Begin by understanding the purpose of the site validation master plan. It is a document that outlines the approach and strategy for ensuring that a site or facility meets the necessary regulatory requirements and industry standards.
02
Identify the key personnel involved in the site validation process. This may include project managers, quality assurance professionals, validation specialists, and other relevant stakeholders.
03
Conduct a comprehensive site assessment to determine the scope of the validation master plan. This involves evaluating the site facilities, equipment, processes, and any relevant documentation that needs to be validated.
04
Define the validation objectives and strategies. This includes establishing clear goals and objectives for the validation process, as well as determining the appropriate validation approach (e.g., risk-based, lifecycle, etc.) and the necessary resources and timeline.
05
Identify and prioritize validation activities. This step involves determining the specific areas and systems that require validation, such as equipment, software, manufacturing processes, cleaning procedures, and analytical methods.
06
Create a validation protocol for each identified validation activity. This includes developing a detailed plan that outlines the validation steps, acceptance criteria, sampling plans, test methods, and documentation requirements.
07
Execute the validation protocols according to the established plan. This may involve performing validation tests, collecting data, analyzing results, and documenting the findings in the validation report.
08
Review and analyze the validation results. Ensure that all acceptance criteria have been met and any deviations or non-conformities have been properly addressed and resolved.
09
Compile the necessary documentation to support the validation process. This includes maintaining accurate and up-to-date records of all validation activities, including protocols, reports, data sheets, and any other relevant documentation.
Who needs a site validation master plan?
A site validation master plan is typically required for regulated industries, such as pharmaceutical, biotechnology, medical devices, and food and beverage manufacturing. Companies operating in these sectors need to ensure that their facilities and processes comply with regulatory requirements, such as Good Manufacturing Practices (GMP), Good Laboratory Practices (GLP), and other relevant standards.
Additionally, organizations that prioritize quality assurance and continuous improvement may also benefit from having a site validation master plan in place. This helps ensure that their facilities and processes are consistently validated and meet the necessary standards for quality and reliability.
Overall, any organization that seeks to ensure the safety, efficacy, and quality of their products or services can benefit from having a site validation master plan. It provides a structured approach to validate facilities, equipment, processes, and systems, reducing risks and ensuring compliance with regulatory requirements.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I modify site validation master plan without leaving Google Drive?
It is possible to significantly enhance your document management and form preparation by combining pdfFiller with Google Docs. This will allow you to generate papers, amend them, and sign them straight from your Google Drive. Use the add-on to convert your site validation master plan into a dynamic fillable form that can be managed and signed using any internet-connected device.
How do I complete site validation master plan online?
Easy online site validation master plan completion using pdfFiller. Also, it allows you to legally eSign your form and change original PDF material. Create a free account and manage documents online.
How do I edit site validation master plan on an iOS device?
Use the pdfFiller mobile app to create, edit, and share site validation master plan from your iOS device. Install it from the Apple Store in seconds. You can benefit from a free trial and choose a subscription that suits your needs.
What is site validation master plan?
A site validation master plan is a documented strategy outlining the approach for validating equipment, facilities, and processes at a specific site.
Who is required to file site validation master plan?
Companies in regulated industries, such as pharmaceuticals and medical devices, are typically required to file a site validation master plan.
How to fill out site validation master plan?
To fill out a site validation master plan, companies need to detail their validation approach, timelines, responsibilities, and procedures for ensuring compliance.
What is the purpose of site validation master plan?
The purpose of a site validation master plan is to ensure that equipment, facilities, and processes are validated correctly to meet regulatory requirements and ensure product quality and safety.
What information must be reported on site validation master plan?
Information such as validation strategies, timelines, risk assessments, validation protocols, and validation reports must be reported on a site validation master plan.
Fill out your site validation master plan online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Site Validation Master Plan is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.