Pre AP Chemistry Unit 7 HW Packet 2010 free printable template
Show details
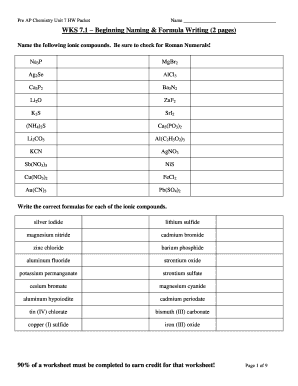
PRE AP Chemistry Unit 7 HW Packet Name WAS 7.1 Beginning Naming & Formula Writing (2 pages) Name the following ionic compounds. Be sure to check for Roman numerals! Na3P MgBr2 Ag2Se AlCl3 Ca3P2 Ba3N2
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign Pre AP Chemistry Unit 7 HW

Edit your Pre AP Chemistry Unit 7 HW form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your Pre AP Chemistry Unit 7 HW form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing Pre AP Chemistry Unit 7 HW online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Check your account. If you don't have a profile yet, click Start Free Trial and sign up for one.
2
Upload a file. Select Add New on your Dashboard and upload a file from your device or import it from the cloud, online, or internal mail. Then click Edit.
3
Edit Pre AP Chemistry Unit 7 HW. Text may be added and replaced, new objects can be included, pages can be rearranged, watermarks and page numbers can be added, and so on. When you're done editing, click Done and then go to the Documents tab to combine, divide, lock, or unlock the file.
4
Save your file. Select it from your records list. Then, click the right toolbar and select one of the various exporting options: save in numerous formats, download as PDF, email, or cloud.
It's easier to work with documents with pdfFiller than you could have believed. You may try it out for yourself by signing up for an account.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
Pre AP Chemistry Unit 7 HW Packet Form Versions
Version
Form Popularity
Fillable & printabley
How to fill out Pre AP Chemistry Unit 7 HW

How to fill out Pre AP Chemistry Unit 7 HW Packet
01
Gather all necessary materials: Pre AP Chemistry Unit 7 HW Packet, a pen or pencil, and a calculator.
02
Read the instructions provided at the top of each page carefully.
03
Refer to your textbook or class notes to understand the concepts covered in Unit 7.
04
Start with the first question and write your answer in the designated space.
05
If required, show your work for calculations to receive full credit.
06
Move on to the next question, ensuring you answer each one completely.
07
Double-check your answers for accuracy.
08
Ensure your packet is neat and legible before submitting.
Who needs Pre AP Chemistry Unit 7 HW Packet?
01
Students enrolled in the Pre AP Chemistry course who need to practice and apply their knowledge from Unit 7.
02
Teachers who want to assess students' understanding of the material covered in Unit 7.
03
Parents or guardians who want to support their child’s learning in chemistry.
Fill
form
: Try Risk Free






People Also Ask about
What are the steps for writing and naming chemical formulas?
write the names of the elements in the order listed in the formula. Use prefixes to indicate the number of each atom. End the name of the second element with -ide. To write the formula of a binary molecular compound, use the prefixes to determine the subscript of each element.
What are Type 1 and Type 2 compounds?
Type I Compounds – The metal present forms only one type of cation. Type II Compounds – The metal present can form two or more cations with different charges.
How are compound formulas written?
To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Then, identify the anion and write down its symbol and charge. Finally, combine the two ions to form an electrically neutral compound.
Why do we need rules in naming and writing chemical compounds?
The primary function of chemical nomenclature is to ensure that a spoken or written chemical name leaves no ambiguity concerning to what chemical compound the name refers. Each chemical name should refer to a single substance.
How do you name three compounds?
Molecular compounds are named with the first element first and then the second element by using the stem of the element name plus the suffix -ide. Numerical prefixes are used to specify the number of atoms in a molecule.
How do you write binary compound formulas?
For a binary ionic compound, a metal will always be the first element in the formula, while a nonmetal will always be the second. The metal cation is named first, followed by the nonmetal anion. Subscripts in the formula do not affect the name. The table below shows three examples.
What are the two major types of binary compounds?
The major types of binary chemical compounds are ionic and covalent. Ionic compounds are made up of a metal cation and a nonmetal anion, while covalent compounds are formed from nonmetal atoms. Examples of ionic binary compounds are: Sodium chloride, NaCl.
How to identify the correct way to name a binary ionic compound?
For binary ionic compounds (ionic compounds that contain only two types of elements), the compounds are named by writing the name of the cation first followed by the name of the anion. For example, KCl, an ionic compound that contains K⁺ and Cl⁻ ions, is named potassium chloride.
How do you name a binary compound?
When naming binary ionic compounds, name the cation first (specifying the charge, if necessary), then the nonmetal anion (element stem + -ide). Do NOT use prefixes to indicate how many of each element is present; this information is implied in the name of the compound.
What are the rules for naming ionic compounds?
When writing an ionic compound, the cation is written first and the anion is written second. The general rule of naming ionic compounds is pretty simple. The rule is: "name of cation" + "name of anion + -ide". So, for NaCl, it would be sodium chloride.
What are the two types of binary compounds?
Binary ionic compounds. Binary covalent/molecular compounds.
How do you name type 3 binary compounds?
Binary Covalent Compounds (Type III) The first element shown in the compound is named as the element (e.g., for CO2, first element is "carbon") The second element shown in the compound is named ing to the anion name, ending in -ide (e.g., for CO2, the second element is named "oxide")
How do you name type 2 binary compounds?
Binary Ionic Compounds (Type II) A monatomic (meaning one-atom) cation takes its name from the name of the element. For example, Cu+ is called Copper(I) and Cu2+ is called Copper(II) in the names of compounds containing these ions. The number in parentheses is the charge of the cation.
How do you name 3 binary compounds?
Binary Covalent Compounds (Type III) The first element shown in the compound is named as the element (e.g., for CO2, first element is "carbon") The second element shown in the compound is named ing to the anion name, ending in -ide (e.g., for CO2, the second element is named "oxide")
What are 2 rules for naming compounds?
Naming compounds Rule one. The element that is furthest left in the periodic table comes first, eg Sodium Chloride/Carbon dioxide. Rule two. If there are only two elements in the compound then the compounds name ends in –ide, eg A compound of copper and sulfur is called copper sulfide. Rule three.
What are the rules in naming and writing compounds?
Molecular compounds are named with the first element first and then the second element by using the stem of the element name plus the suffix -ide. Numerical prefixes are used to specify the number of atoms in a molecule.
What are Type 1 and 2 binary ionic compounds?
Type 1 binary ionic compounds are those in which the cation has only one form, or charge. Type 2 binary ionic compounds are those in which the cation can have multiple forms. Additionally, binary ionic compounds containing polyatomic ions have another distinct set of naming rules.
How do you name binary compounds and acids?
Naming Binary acids (in aqueous form) The most common binary acids contain a halogen. The acid name begins with the prefix hydro-. followed by the base name of the anion, followed by the suffix -ic. Formula for naming acids: Hydro- and Base name of nonmetal and -ic + acid.
How do you name a binary molecule compound?
Naming binary molecular compounds is really quite easy. The first element is given its element name; the second is given its root (hydr, bor, carb, ox, fluor, etc.) followed by ide. For example, HCl is hydrogen chloride, and H2Se is hydrogen selenide.
How are formulas written for binary ionic compounds?
For a binary ionic compound, a metal will always be the first element in the formula, while a nonmetal will always be the second. The metal cation is named first, followed by the nonmetal anion. Subscripts in the formula do not affect the name. The table below shows three examples.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I manage my Pre AP Chemistry Unit 7 HW directly from Gmail?
You may use pdfFiller's Gmail add-on to change, fill out, and eSign your Pre AP Chemistry Unit 7 HW as well as other documents directly in your inbox by using the pdfFiller add-on for Gmail. pdfFiller for Gmail may be found on the Google Workspace Marketplace. Use the time you would have spent dealing with your papers and eSignatures for more vital tasks instead.
How do I fill out the Pre AP Chemistry Unit 7 HW form on my smartphone?
You can easily create and fill out legal forms with the help of the pdfFiller mobile app. Complete and sign Pre AP Chemistry Unit 7 HW and other documents on your mobile device using the application. Visit pdfFiller’s webpage to learn more about the functionalities of the PDF editor.
Can I edit Pre AP Chemistry Unit 7 HW on an iOS device?
Create, edit, and share Pre AP Chemistry Unit 7 HW from your iOS smartphone with the pdfFiller mobile app. Installing it from the Apple Store takes only a few seconds. You may take advantage of a free trial and select a subscription that meets your needs.
What is Pre AP Chemistry Unit 7 HW Packet?
The Pre AP Chemistry Unit 7 HW Packet is a collection of assignments and exercises designed for students to reinforce concepts learned in Unit 7 of the Pre AP Chemistry curriculum.
Who is required to file Pre AP Chemistry Unit 7 HW Packet?
Students enrolled in the Pre AP Chemistry course are required to file the Unit 7 HW Packet as part of their coursework.
How to fill out Pre AP Chemistry Unit 7 HW Packet?
To fill out the Pre AP Chemistry Unit 7 HW Packet, students should carefully read each question, provide their responses in the designated spaces, and ensure all required sections are completed before submission.
What is the purpose of Pre AP Chemistry Unit 7 HW Packet?
The purpose of the Pre AP Chemistry Unit 7 HW Packet is to facilitate practice and application of key chemistry concepts, assess student understanding, and prepare students for assessments.
What information must be reported on Pre AP Chemistry Unit 7 HW Packet?
Students must report their name, the date, and responses to the assigned problems. Additionally, any specific instructions or additional notes from the teacher may also need to be included.
Fill out your Pre AP Chemistry Unit 7 HW online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Pre AP Chemistry Unit 7 HW is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.