
Get the free Institutional Review Board for the Protection of Human Subjects - niagara
Show details
The 2014 Great Guatemalan Challenge, an eight-day backpacking trip January 512, 2014. Who Can Go? This trip is designed for adults, age 21 and older, who are interested in an adventure and a challenge.
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign institutional review board for
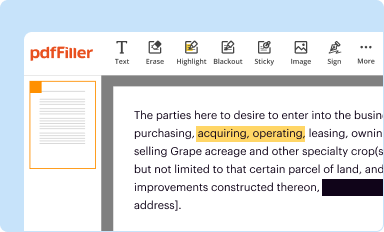
Edit your institutional review board for form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.
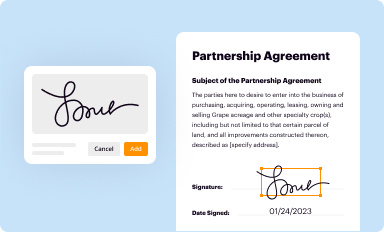
Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your institutional review board for form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing institutional review board for online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Create an account. Begin by choosing Start Free Trial and, if you are a new user, establish a profile.
2
Upload a document. Select Add New on your Dashboard and transfer a file into the system in one of the following ways: by uploading it from your device or importing from the cloud, web, or internal mail. Then, click Start editing.
3
Edit institutional review board for. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Save your file. Choose it from the list of records. Then, shift the pointer to the right toolbar and select one of the several exporting methods: save it in multiple formats, download it as a PDF, email it, or save it to the cloud.
With pdfFiller, it's always easy to work with documents. Try it out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out institutional review board for
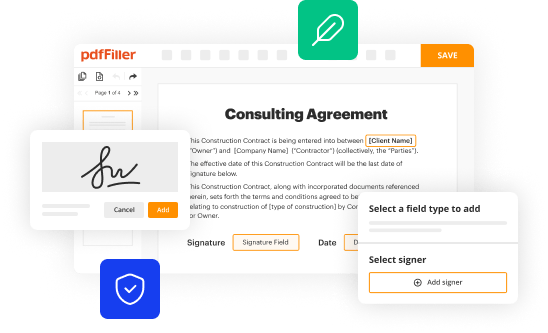
Point by point on how to fill out institutional review board (IRB) forms:
01
Begin by familiarizing yourself with the specific requirements of the IRB. Each institution may have slightly different protocols and forms, so it's essential to understand the guidelines before starting the application process.
02
Gather all the necessary information and documents. This usually includes details about the research project, such as the purpose, methods, anticipated risks, and potential benefits. You may also need to provide information about the research team, funding, and any conflicts of interest.
03
Start filling out the IRB application form by providing your contact information, including your name, title, department, and institutional affiliation. Make sure to double-check the accuracy of this information to avoid any delays in the review process.
04
Describe your research project thoroughly. This includes providing a clear, concise, and detailed explanation of the study's objectives, methodology, and the population under investigation. You should also outline any potential risks to participants and the steps you will take to mitigate them.
05
Address the ethical considerations. In this section, you need to explain how you will ensure the welfare and rights of participants, including informed consent procedures, confidentiality measures, and how you plan to minimize any harm or discomfort.
06
Include any supporting documents required by the IRB, such as consent forms, recruitment materials, questionnaires, or surveys. Make sure these documents are properly formatted, clearly labeled, and comply with any specific requirements set forth by the IRB.
07
After completing the paperwork, review the entire application thoroughly to ensure accuracy and consistency. Proofread for any typos or grammatical errors, as they can give a negative impression of your professionalism.
08
Submit your application to the IRB according to the institution's guidelines, either through an online platform or by physically delivering the forms to the designated office. Keep copies of all the documents you submitted for your records.
Who needs an institutional review board (IRB) for?
01
Researchers who intend to conduct studies involving human subjects are generally required to obtain IRB approval. This includes academics, scientists, healthcare professionals, and undergraduate or graduate students.
02
Institutions, such as universities, hospitals, research centers, or any organization receiving federal funding, often require researchers to obtain IRB approval before starting their projects. IRBs serve as a regulatory body that ensures the protection of participants' rights, ethical conduct, and adherence to applicable laws and regulations.
03
Various fields of research, including medical trials, psychological studies, social sciences, anthropology, education, and public health, require IRB review. Regardless of the specific research area, if it involves human subjects, IRB approval is typically necessary to ensure the ethical treatment and welfare of the participants.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is institutional review board for?
The institutional review board is responsible for ensuring the protection of the rights, safety, and wellbeing of human subjects participating in research studies.
Who is required to file institutional review board for?
Researchers conducting studies involving human subjects are required to file an institutional review board.
How to fill out institutional review board for?
Researchers need to provide detailed information about their study, including the research protocol, potential risks and benefits, and informed consent procedures.
What is the purpose of institutional review board for?
The purpose of the institutional review board is to review and approve research studies involving human subjects to ensure ethical standards are met.
What information must be reported on institutional review board for?
Researchers must report detailed information about the study design, participant recruitment, data collection procedures, and risk assessment.
How can I send institutional review board for to be eSigned by others?
Once you are ready to share your institutional review board for, you can easily send it to others and get the eSigned document back just as quickly. Share your PDF by email, fax, text message, or USPS mail, or notarize it online. You can do all of this without ever leaving your account.
Can I create an eSignature for the institutional review board for in Gmail?
It's easy to make your eSignature with pdfFiller, and then you can sign your institutional review board for right from your Gmail inbox with the help of pdfFiller's add-on for Gmail. This is a very important point: You must sign up for an account so that you can save your signatures and signed documents.
How do I edit institutional review board for straight from my smartphone?
You can easily do so with pdfFiller's apps for iOS and Android devices, which can be found at the Apple Store and the Google Play Store, respectively. You can use them to fill out PDFs. We have a website where you can get the app, but you can also get it there. When you install the app, log in, and start editing institutional review board for, you can start right away.
Fill out your institutional review board for online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Institutional Review Board For is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















