India Investigational Medicinal Product Dossier 2016 free printable template
Show details
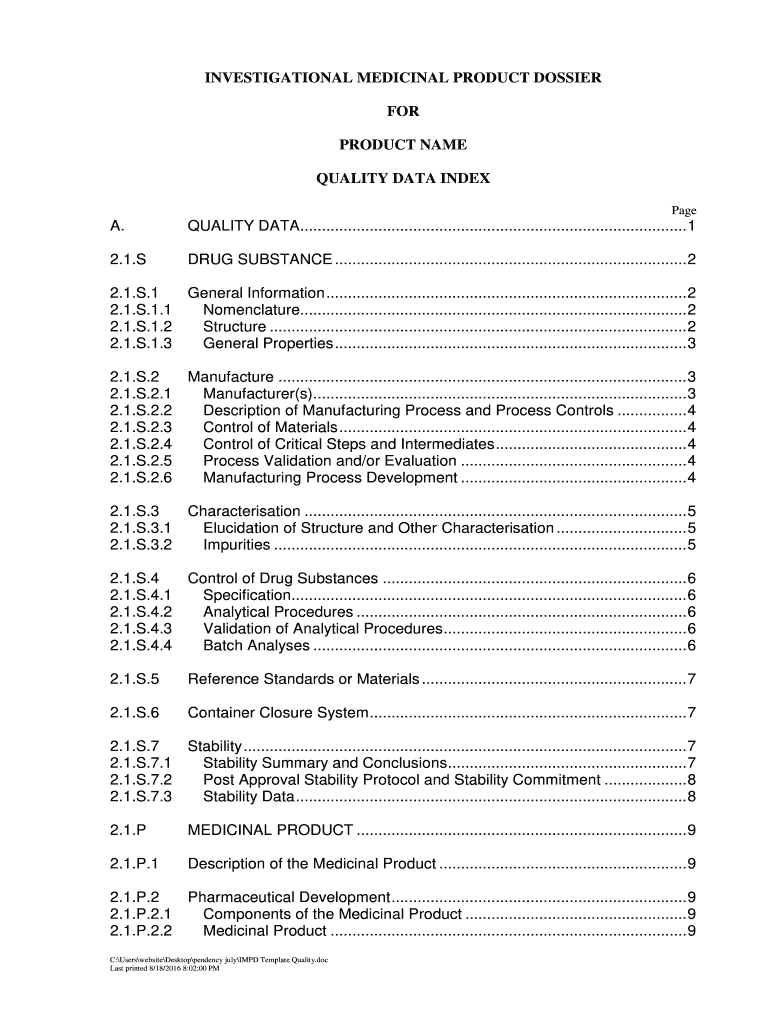
INVESTIGATIONAL MEDICINAL PRODUCT DOSSIER
FOR
PRODUCT NAME
QUALITY DATA INDEX
Page. QUALITY DATA......................................................................................... 12.1.DRUG
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign India Investigational Medicinal Product Dossier

Edit your India Investigational Medicinal Product Dossier form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your India Investigational Medicinal Product Dossier form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing India Investigational Medicinal Product Dossier online
Use the instructions below to start using our professional PDF editor:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit India Investigational Medicinal Product Dossier. Replace text, adding objects, rearranging pages, and more. Then select the Documents tab to combine, divide, lock or unlock the file.
4
Save your file. Select it from your list of records. Then, move your cursor to the right toolbar and choose one of the exporting options. You can save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud, among other things.
pdfFiller makes working with documents easier than you could ever imagine. Register for an account and see for yourself!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
India Investigational Medicinal Product Dossier Form Versions
Version
Form Popularity
Fillable & printabley
How to fill out India Investigational Medicinal Product Dossier

How to fill out India Investigational Medicinal Product Dossier
01
Gather all relevant data on the investigational medicinal product including its composition, manufacturing process, and control methods.
02
Prepare the clinical trial protocol that outlines the study objectives, design, methodology, and statistical considerations.
03
Compile pharmacological and toxicological data to support the safety and efficacy of the product.
04
Include a detailed summary of previous human experience, if available, or relevant animal studies.
05
Prepare packaging and labeling information as per regulatory requirements.
06
Ensure that all documentation is signed and dated by the responsible individual.
07
Organize the complete dossier according to the guidelines provided by the Central Drugs Standard Control Organization (CDSCO) of India.
08
Submit the dossier along with the application for obtaining permission to conduct clinical trials.
Who needs India Investigational Medicinal Product Dossier?
01
Pharmaceutical companies looking to conduct clinical trials in India.
02
Regulatory affairs professionals involved in the submission of investigational products.
03
Researchers and sponsors planning to test new drugs or therapies on human subjects in India.
04
Any organization or entity requiring approval from CDSCO for investigational medicinal products.
Fill
form
: Try Risk Free






People Also Ask about
What is the full form of IMPD in pharma?
The Investigational Medicinal Product Dossier (IMPD) is a central piece of Investigational Medicinal Product (IMP) related documents required for approval of clinical trials by the competent authorities in the EU.
What is the difference between Nimp and IMP?
A Non Investigational Medicinal Product (NIMP) is a medicinal product which is not classed as an IMP in a trial, but may be taken by subjects during the trial.
What does IMP stand for Pharma?
Investigational Medicinal Product (IMP)
What is a investigational medicinal product dossier?
An Investigational Medicinal Product Dossier (IMPD) contains data on the quality, production and control of the medicinal product being researched. The IMPD consists of 2 parts: The Quality section with information on the active medicinal product, placebo and reference medicine (if applicable).
What is the US equivalent of Impd?
Investigational New Drug (IND) applications are the equivalent in the United States.
What is the meaning of Impd?
An Investigational Medicinal Product Dossier (IMPD) contains data on the quality, production and control of the medicinal product being researched.
Does FDA require IMPD?
An IMPD is required for IMPs to be used in a clinical study, regardless of whether it is the test product itself, a reference product already authorised or a placebo.
What is considered investigational medicinal product?
Regulation (EU) No 536/2014 Article 2 (5) defines an IMP as “a medicinal. 47. product which is being tested or used as a reference, including as a placebo, in a. 48. clinical trial”.
What is an IMPD submission?
The Investigational Medicinal Product Dossier (IMPD) is a central piece of Investigational Medicinal Product (IMP) related documents required for approval of clinical trials by the competent authorities in the EU.
What is the Impd equivalent in the US?
IMPDs are submitted as part of the Clinical Trial Application Dossier, as the basis for approval of clinical trials by competent regulatory authorities within the European Union. Investigational New Drug (IND) applications are the equivalent in the United States.
What are the Labelling requirements for investigational medicinal products?
§ 312.6 Labeling of an investigational new drug. (b) The label or labeling of an investigational new drug shall not bear any statement that is false or misleading in any particular and shall not represent that the investigational new drug is safe or effective for the purposes for which it is being investigated.
What is the difference between IND and IMPD?
What is IND and IMPD? IND (Investigational New Drug) and IMPD are regulatory documents submitted to Competent Authority (IND to FDA, IMPD to EMA) for an investigational medicinal drug for approval to begin human clinical trials.
What are the contents of investigational medicinal product dossier?
An Investigational Medicinal Product Dossier (IMPD) contains data on the quality, production and control of the medicinal product being researched. The IMPD consists of 2 parts: The Quality section with information on the active medicinal product, placebo and reference medicine (if applicable).
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I edit India Investigational Medicinal Product Dossier from Google Drive?
Simplify your document workflows and create fillable forms right in Google Drive by integrating pdfFiller with Google Docs. The integration will allow you to create, modify, and eSign documents, including India Investigational Medicinal Product Dossier, without leaving Google Drive. Add pdfFiller’s functionalities to Google Drive and manage your paperwork more efficiently on any internet-connected device.
How do I edit India Investigational Medicinal Product Dossier online?
With pdfFiller, you may not only alter the content but also rearrange the pages. Upload your India Investigational Medicinal Product Dossier and modify it with a few clicks. The editor lets you add photos, sticky notes, text boxes, and more to PDFs.
How do I complete India Investigational Medicinal Product Dossier on an iOS device?
pdfFiller has an iOS app that lets you fill out documents on your phone. A subscription to the service means you can make an account or log in to one you already have. As soon as the registration process is done, upload your India Investigational Medicinal Product Dossier. You can now use pdfFiller's more advanced features, like adding fillable fields and eSigning documents, as well as accessing them from any device, no matter where you are in the world.
What is India Investigational Medicinal Product Dossier?
The India Investigational Medicinal Product Dossier (IMPD) is a comprehensive document that provides detailed information about an investigational medicinal product, including its manufacturing process, quality control, and clinical data supporting its safety and efficacy for use in clinical trials.
Who is required to file India Investigational Medicinal Product Dossier?
Sponsors, typically pharmaceutical companies or research organizations initiating clinical trials in India, are required to file the India Investigational Medicinal Product Dossier with the regulatory authorities.
How to fill out India Investigational Medicinal Product Dossier?
Filling out the India Investigational Medicinal Product Dossier involves providing structured information in specified sections, including product details, manufacturing processes, stability data, pharmacology, toxicology, and clinical trial protocols, ensuring compliance with regulatory guidelines.
What is the purpose of India Investigational Medicinal Product Dossier?
The purpose of the India Investigational Medicinal Product Dossier is to provide regulatory authorities with the necessary scientific data to evaluate the safety and efficacy of the investigational medicinal product, thereby enabling the approval for clinical trials.
What information must be reported on India Investigational Medicinal Product Dossier?
The India Investigational Medicinal Product Dossier must report detailed information including the product composition, manufacturing and quality control processes, stability data, pharmacodynamics, pharmacokinetics, toxicological information, and clinical study protocols.
Fill out your India Investigational Medicinal Product Dossier online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

India Investigational Medicinal Product Dossier is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.























