Get the free urinalysis quality control log
Show details
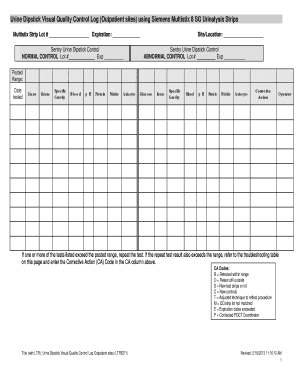
Urine Dipstick Visual Quality Control Log (Outpatient sites) using Siemens Multisite 8 SG Urinalysis Strips Multisite Strip Lot # Expiration: Sentry Urine Dipstick Control NORMAL CONTROL Lot # Exp
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign urinalysis quality control log

Edit your urinalysis quality control log form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your urinalysis quality control log form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing urinalysis quality control log online
To use our professional PDF editor, follow these steps:
1
Log in to account. Start Free Trial and sign up a profile if you don't have one.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit urinalysis quality control log. Rearrange and rotate pages, insert new and alter existing texts, add new objects, and take advantage of other helpful tools. Click Done to apply changes and return to your Dashboard. Go to the Documents tab to access merging, splitting, locking, or unlocking functions.
4
Save your file. Select it from your records list. Then, click the right toolbar and select one of the various exporting options: save in numerous formats, download as PDF, email, or cloud.
pdfFiller makes working with documents easier than you could ever imagine. Try it for yourself by creating an account!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out urinalysis quality control log

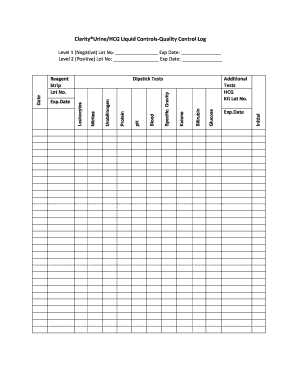
How to fill out urinalysis quality control log:
01
Write the date of the quality control test in the designated column.
02
Record the lot number or identifier of the control material used.
03
Note down the expiration date or date of opening the control material.
04
Fill in the target value or expected result for each analyte being tested.
05
Perform the urinalysis test using the control material according to the manufacturer's instructions.
06
Record the actual result obtained for each analyte in the appropriate column.
07
Compare the actual results with the target values and assess if they fall within an acceptable range.
08
Document any corrective actions taken if there are deviations from the target values.
09
Sign and date the log to indicate completion of the quality control testing.
Who needs urinalysis quality control log:
01
Clinical laboratories performing urinalysis tests.
02
Medical professionals and technologists responsible for quality assurance in urinalysis.
03
Institutions or organizations following regulatory requirements for maintaining accurate and reliable urinalysis results.
Fill
form
: Try Risk Free






People Also Ask about
How do you analyze urine test results?
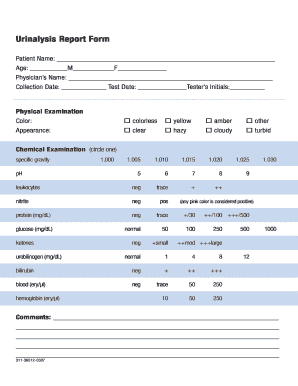
Dipstick test Acidity (pH). The pH level indicates the amount of acid in urine. Concentration. A measure of concentration shows how concentrated the particles are in your urine. Protein. Low levels of protein in urine are typical. Sugar. Ketones. Bilirubin. Evidence of infection. Blood.
What are some examples of abnormal findings in a urinalysis?
A high urine pH may indicate conditions including kidney issues and a urinary tract infection (UTI). A low urine pH may indicate conditions including diabetes-related ketoacidosis and diarrhea. Ketones urine test: Ketones build up when your body has to break down fats and fatty acids to use as fuel for energy.
How do you document normal urine output?
To calculate the rate of urine output, divide the volume of urine produced by the number of hours that have elapsed since the bag/chamber was last emptied (e.g. 80ml over 2 hours = 40ml/hour).
How do I document my urinalysis results?
Documenting the urinalysis results in the notes Document the time and date that the urinalysis was performed (as this may be significantly different from the time you are documenting). Write the indication for the urinalysis (e.g. dysuria). Inspect the urine and document the following characteristics of the urine:
What is quality control in urinalysis?
Running daily quality control for your urine analyzer is intended to identify any problems that could impact the instrument's ability to accurately detect parameters in a patient sample.
Our user reviews speak for themselves
Read more or give pdfFiller a try to experience the benefits for yourself
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I send urinalysis quality control log for eSignature?
urinalysis quality control log is ready when you're ready to send it out. With pdfFiller, you can send it out securely and get signatures in just a few clicks. PDFs can be sent to you by email, text message, fax, USPS mail, or notarized on your account. You can do this right from your account. Become a member right now and try it out for yourself!
How do I make changes in urinalysis quality control log?
The editing procedure is simple with pdfFiller. Open your urinalysis quality control log in the editor, which is quite user-friendly. You may use it to blackout, redact, write, and erase text, add photos, draw arrows and lines, set sticky notes and text boxes, and much more.
How do I edit urinalysis quality control log on an Android device?
You can. With the pdfFiller Android app, you can edit, sign, and distribute urinalysis quality control log from anywhere with an internet connection. Take use of the app's mobile capabilities.
What is urinalysis quality control log?
A urinalysis quality control log is a record-keeping document used to track the quality control procedures and results for urinalysis testing, ensuring accuracy and reliability of the test results.
Who is required to file urinalysis quality control log?
Laboratory personnel, specifically those involved in performing urinalysis tests, are required to file and maintain the urinalysis quality control log.
How to fill out urinalysis quality control log?
To fill out a urinalysis quality control log, record the date and time of testing, the testing procedures followed, results of quality control tests, names of personnel conducting the test, and any corrective actions taken if quality control standards are not met.
What is the purpose of urinalysis quality control log?
The purpose of the urinalysis quality control log is to document the consistency and reliability of testing processes, identify any deviations from standard procedures, and ensure compliance with regulatory standards.
What information must be reported on urinalysis quality control log?
The information that must be reported includes the date and time of testing, the type of quality control materials used, test results, the identity of the personnel performing the tests, and any corrective actions taken.
Fill out your urinalysis quality control log online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Urinalysis Quality Control Log is not the form you're looking for?Search for another form here.
Relevant keywords
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.