Get the free MAY 23 2011 510k SUMMARY for EG VI Pro Self Monitoring - accessdata fda
Show details
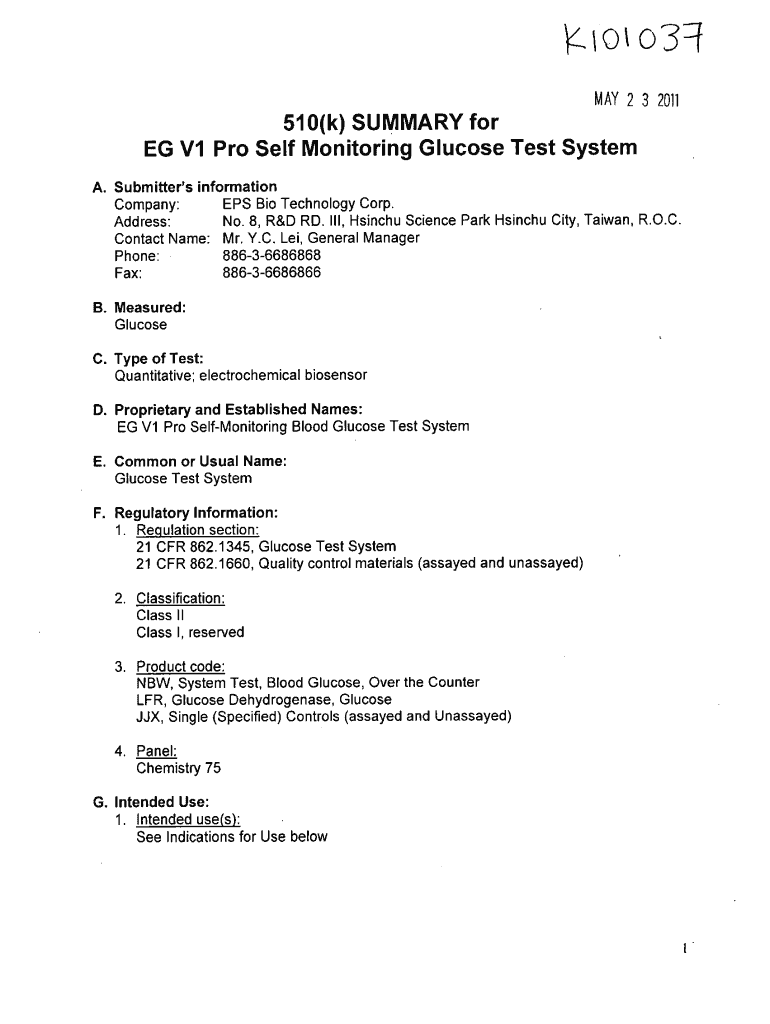
MAY 23, 2011 510(k) SUMMARY for EG VI Pro Self Monitoring Glucose Test System A. Submitter's information EPS BIA Technology Corp. Company: Since Science Park Since City, Taiwan, R.O.C. No. 8, R&D
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign may 23 2011 510k

Edit your may 23 2011 510k form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your may 23 2011 510k form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit may 23 2011 510k online
Follow the steps down below to take advantage of the professional PDF editor:
1
Log in. Click Start Free Trial and create a profile if necessary.
2
Upload a file. Select Add New on your Dashboard and upload a file from your device or import it from the cloud, online, or internal mail. Then click Edit.
3
Edit may 23 2011 510k. Add and change text, add new objects, move pages, add watermarks and page numbers, and more. Then click Done when you're done editing and go to the Documents tab to merge or split the file. If you want to lock or unlock the file, click the lock or unlock button.
4
Get your file. Select the name of your file in the docs list and choose your preferred exporting method. You can download it as a PDF, save it in another format, send it by email, or transfer it to the cloud.
With pdfFiller, it's always easy to work with documents.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out may 23 2011 510k

How to fill out May 23, 2011 510(k):
01
Begin by obtaining the necessary form. The May 23, 2011 510(k) is a specific type of premarket notification form that medical device manufacturers need to complete and submit to the U.S. Food and Drug Administration (FDA).
02
Provide the required general information at the top of the form, including the submitter's name, address, phone number, and email.
03
Identify the manufacturer's name, address, and contact information. This should match the information provided in the establishment registration with the FDA.
04
Specify the proprietary name and intended use of the medical device for which the 510(k) is being filed. Include a brief description of the device and explain how it is similar to or different from any previously cleared devices.
05
Include the medical device's classification information, which helps the FDA determine the level of regulatory control necessary for its approval. This can be found by referring to the FDA's device classification database.
06
Provide a substantial equivalence statement, comparing the new device to a legally marketed device that is already cleared by the FDA. This statement should demonstrate that the new device is as safe and effective as the predicate device.
07
Include any test data or scientific evidence supporting the substantial equivalence claim. This may involve providing information on the device's design, performance, materials, and clinical studies. It's important to include objective and reliable data to strengthen the application.
08
Attach any relevant labeling or user instructions for the device, ensuring that they comply with FDA regulations and guidelines.
09
Prepare a summary of the 510(k) submission, highlighting the key points and conclusions. This summary should convey a clear and concise message regarding the safety and effectiveness of the device.
10
Double-check all the information provided, ensuring its accuracy and completeness. It's a good practice to review the submission multiple times to avoid any errors or omissions.
Who needs May 23, 2011 510(k)?
01
Medical device manufacturers planning to introduce a new device to the U.S. market that does not qualify for an exemption or a different regulatory pathway may need the May 23, 2011 510(k).
02
Manufacturers who have made modifications to an existing device that could significantly affect its safety or efficacy may also require the May 23, 2011 510(k).
03
Companies seeking to obtain clearance for their medical devices from the FDA to ensure compliance with regulatory requirements would benefit from the May 23, 2011 510(k) process.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I send may 23 2011 510k for eSignature?
When your may 23 2011 510k is finished, send it to recipients securely and gather eSignatures with pdfFiller. You may email, text, fax, mail, or notarize a PDF straight from your account. Create an account today to test it.
How do I edit may 23 2011 510k on an Android device?
Yes, you can. With the pdfFiller mobile app for Android, you can edit, sign, and share may 23 2011 510k on your mobile device from any location; only an internet connection is needed. Get the app and start to streamline your document workflow from anywhere.
How do I complete may 23 2011 510k on an Android device?
Complete may 23 2011 510k and other documents on your Android device with the pdfFiller app. The software allows you to modify information, eSign, annotate, and share files. You may view your papers from anywhere with an internet connection.
What is may 23 510k summary?
The May 23 510(k) summary is a summary of a premarket notification submission to the FDA that demonstrates a medical device is substantially equivalent to a legally marketed device.
Who is required to file may 23 510k summary?
Manufacturers or distributors of medical devices who are seeking FDA approval to market their devices are required to file a 510(k) summary.
How to fill out may 23 510k summary?
The May 23 510(k) summary should be filled out with detailed information about the device, including its intended use, technology, safety, and performance data.
What is the purpose of may 23 510k summary?
The purpose of the May 23 510(k) summary is to demonstrate to the FDA that a new medical device is substantially equivalent to a device that is already legally marketed.
What information must be reported on may 23 510k summary?
The May 23 510(k) summary must include information about the device, its intended use, design, materials, performance data, and any applicable clinical studies.
Fill out your may 23 2011 510k online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

May 23 2011 510k is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.



















