Get the free ANALYTICAL METHOD DEVELOPMENT AND VALIDATION OF LAFUTIDINE IN TABLET DOSAGE FORM BY ...
Show details
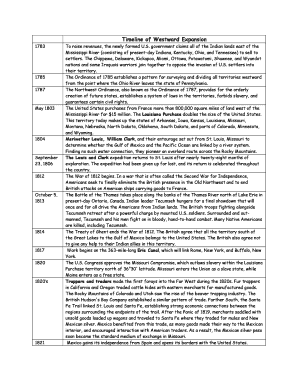
International Journal of Chester Research CODE(USA): ICGG ISSN : 0974-4290 Vol. 3, No.3, pp 1403-1407, July-Sept 2011 Analytical Method Development and Validation of Famotidine in Tablet dosage form
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign analytical method development and

Edit your analytical method development and form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your analytical method development and form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing analytical method development and online
In order to make advantage of the professional PDF editor, follow these steps below:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Upload a file. Select Add New on your Dashboard and upload a file from your device or import it from the cloud, online, or internal mail. Then click Edit.
3
Edit analytical method development and. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Get your file. Select the name of your file in the docs list and choose your preferred exporting method. You can download it as a PDF, save it in another format, send it by email, or transfer it to the cloud.
With pdfFiller, it's always easy to work with documents. Try it!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out analytical method development and

How to fill out analytical method development and:
01
Start by clearly defining the objectives of the analytical method development. Understand the purpose and requirements of the analysis that needs to be performed.
02
Gather all the necessary information and materials for the development process. This may include the samples, standards, reagents, instruments, and any specific protocols or guidelines.
03
Conduct a thorough literature review to understand the existing methods and techniques related to the analysis. This will help in determining the appropriate approach for developing the analytical method.
04
Plan and design the experiments for method development. Consider factors like sample preparation, calibration curves, analytical techniques, detection limits, accuracy, precision, and selectivity.
05
Perform the experiments according to the planned design. Analyze the obtained results and make necessary adjustments or optimizations in the method parameters if required.
06
Validate the developed method to ensure its reliability, accuracy, and precision. Follow validation guidelines and perform validation experiments using appropriate statistical tools and acceptance criteria.
07
Document all the steps taken during the analytical method development process, including the procedures, data, observations, and any modifications made. This documentation will serve as a reference for future use and ensure traceability.
08
Finally, review and finalize the developed analytical method before its implementation. Seek feedback and inputs from relevant stakeholders to ensure its suitability and effectiveness.
Who needs analytical method development and:
01
Researchers and scientists working in various fields such as pharmaceuticals, chemistry, biochemistry, and environmental science require analytical method development to establish reliable and accurate procedures for analyzing their samples.
02
Quality control and quality assurance departments in industries rely on analytical method development to ensure the quality and consistency of their products. This includes sectors like food and beverages, pharmaceuticals, cosmetics, and manufacturing.
03
Regulatory bodies and agencies responsible for monitoring and enforcing quality standards often require analytical method development to ensure compliance and accurate measurements.
04
Academic institutions and research laboratories utilize analytical method development for various research and educational purposes. This helps in advancing scientific understanding and training future scientists in analytical techniques.
05
Contract research organizations (CROs) and analytical service providers offer analytical method development as a specialized service to cater to the needs of clients who lack the necessary expertise or resources.
In summary, analytical method development is essential for achieving accurate and reliable analytical results across various industries and research domains.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is analytical method development and?
Analytical method development is the process of creating and optimizing methods for the analysis of a compound or mixture.
Who is required to file analytical method development and?
Analytical method development is typically required to be filed by researchers, scientists, and laboratories involved in the development of new compounds or products.
How to fill out analytical method development and?
Analytical method development is filled out by providing detailed information about the method used for analysis, including parameters, conditions, and validation procedures.
What is the purpose of analytical method development and?
The purpose of analytical method development is to ensure accurate and reliable analysis of compounds or products.
What information must be reported on analytical method development and?
Information such as method parameters, validation data, analytical procedures, and results must be reported on analytical method development.
How can I edit analytical method development and on a smartphone?
The best way to make changes to documents on a mobile device is to use pdfFiller's apps for iOS and Android. You may get them from the Apple Store and Google Play. Learn more about the apps here. To start editing analytical method development and, you need to install and log in to the app.
How do I fill out analytical method development and using my mobile device?
You can quickly make and fill out legal forms with the help of the pdfFiller app on your phone. Complete and sign analytical method development and and other documents on your mobile device using the application. If you want to learn more about how the PDF editor works, go to pdfFiller.com.
Can I edit analytical method development and on an iOS device?
No, you can't. With the pdfFiller app for iOS, you can edit, share, and sign analytical method development and right away. At the Apple Store, you can buy and install it in a matter of seconds. The app is free, but you will need to set up an account if you want to buy a subscription or start a free trial.
Fill out your analytical method development and online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Analytical Method Development And is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.