Get the free New Medicines Committee Briefing
Show details
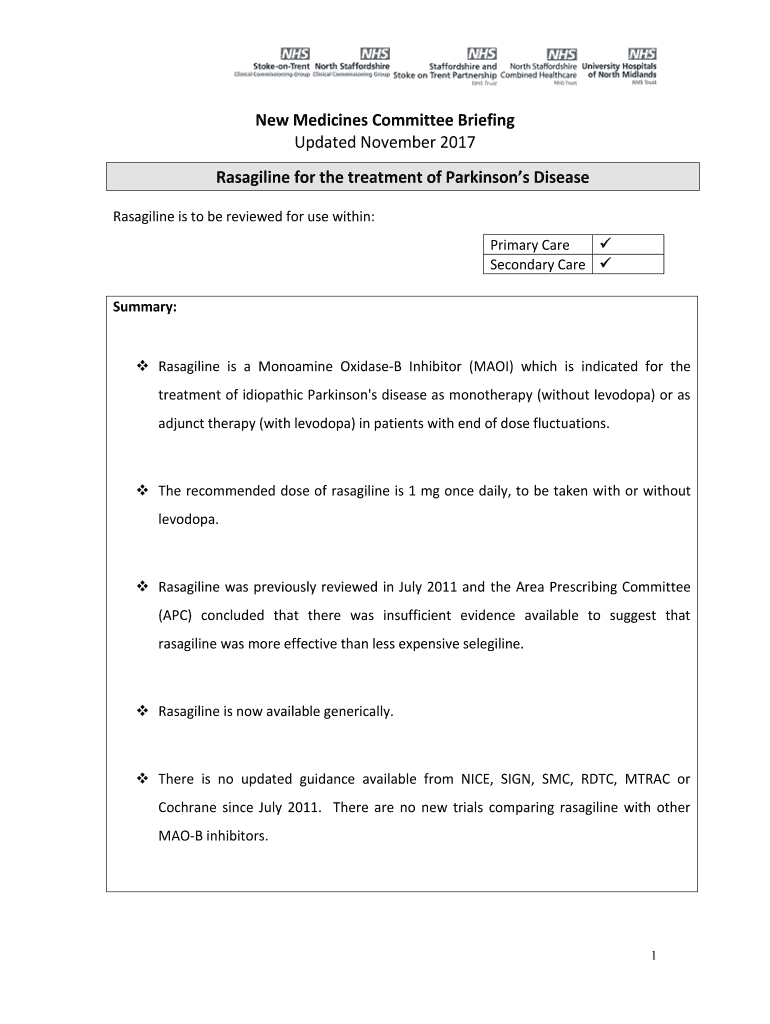
New Medicines Committee Briefing Updated November 2017 Rosaline for the treatment of Parkinson's Disease Rosaline is to be reviewed for use within: Primary Care Secondary Care Summary: Rosaline is
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign new medicines committee briefing

Edit your new medicines committee briefing form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your new medicines committee briefing form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit new medicines committee briefing online
Use the instructions below to start using our professional PDF editor:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit new medicines committee briefing. Add and replace text, insert new objects, rearrange pages, add watermarks and page numbers, and more. Click Done when you are finished editing and go to the Documents tab to merge, split, lock or unlock the file.
4
Get your file. When you find your file in the docs list, click on its name and choose how you want to save it. To get the PDF, you can save it, send an email with it, or move it to the cloud.
It's easier to work with documents with pdfFiller than you can have believed. Sign up for a free account to view.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out new medicines committee briefing

How to fill out new medicines committee briefing
01
To fill out a new medicines committee briefing, follow these steps:
02
Start by entering the essential details of the medicine such as its name, dosage form, and strength.
03
Provide a brief description of the medicine, including its purpose, intended use, and target patient population.
04
Include the known or expected benefits of the medicine, highlighting its effectiveness and potential impact on patient outcomes.
05
Discuss any potential risks or side effects associated with the medicine, along with the proposed mitigation strategies.
06
Outline the proposed dosing regimen, including dosage instructions, frequency, and duration of treatment.
07
Provide supporting evidence from clinical trials or studies that demonstrate the safety and efficacy of the medicine.
08
Include any relevant information on the manufacturing process, quality control, and stability of the medicine.
09
Discuss the proposed labeling and packaging of the medicine, ensuring it complies with regulatory guidelines.
10
Summarize any ongoing or previous regulatory actions or approvals related to the medicine.
11
Conclude the briefing with a recommendation for the committee, stating whether the medicine should be approved for use or not.
12
Ensure the briefing is clear, concise, well-organized, and supported by relevant data and scientific evidence.
13
Review and proofread the briefing for any errors or inconsistencies before submission to the committee.
Who needs new medicines committee briefing?
01
The new medicines committee briefing is required for individuals or organizations involved in the process of reviewing and approving new medicines.
02
This includes regulatory agencies, such as the Food and Drug Administration (FDA), European Medicines Agency (EMA), or national health authorities.
03
Healthcare professionals, including physicians, pharmacists, and researchers, may also need this briefing to make informed decisions on prescribing or using new medicines.
04
Pharmaceutical companies and drug manufacturers may prepare this briefing to seek regulatory approval for their new medicines.
05
Additionally, patient advocacy groups, healthcare policymakers, and other stakeholders may require this briefing to evaluate the safety, efficacy, and cost-effectiveness of new medicines.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I send new medicines committee briefing to be eSigned by others?
When you're ready to share your new medicines committee briefing, you can swiftly email it to others and receive the eSigned document back. You may send your PDF through email, fax, text message, or USPS mail, or you can notarize it online. All of this may be done without ever leaving your account.
Where do I find new medicines committee briefing?
The premium pdfFiller subscription gives you access to over 25M fillable templates that you can download, fill out, print, and sign. The library has state-specific new medicines committee briefing and other forms. Find the template you need and change it using powerful tools.
How do I complete new medicines committee briefing online?
pdfFiller has made it simple to fill out and eSign new medicines committee briefing. The application has capabilities that allow you to modify and rearrange PDF content, add fillable fields, and eSign the document. Begin a free trial to discover all of the features of pdfFiller, the best document editing solution.
What is new medicines committee briefing?
New medicines committee briefing is a document that provides an overview of a new medication or treatment to the medicines committee for evaluation and approval.
Who is required to file new medicines committee briefing?
Medical companies and pharmaceutical companies are required to file new medicines committee briefing.
How to fill out new medicines committee briefing?
New medicines committee briefing should be filled out with detailed information about the medication or treatment, including its proposed uses, side effects, and efficacy.
What is the purpose of new medicines committee briefing?
The purpose of new medicines committee briefing is to present new medications or treatments to the committee for evaluation and approval.
What information must be reported on new medicines committee briefing?
Information such as proposed uses, side effects, efficacy, and any relevant clinical trial data must be reported on new medicines committee briefing.
Fill out your new medicines committee briefing online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

New Medicines Committee Briefing is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















