Get the free Supplemental New Drug Application
Show details
NDA 21330
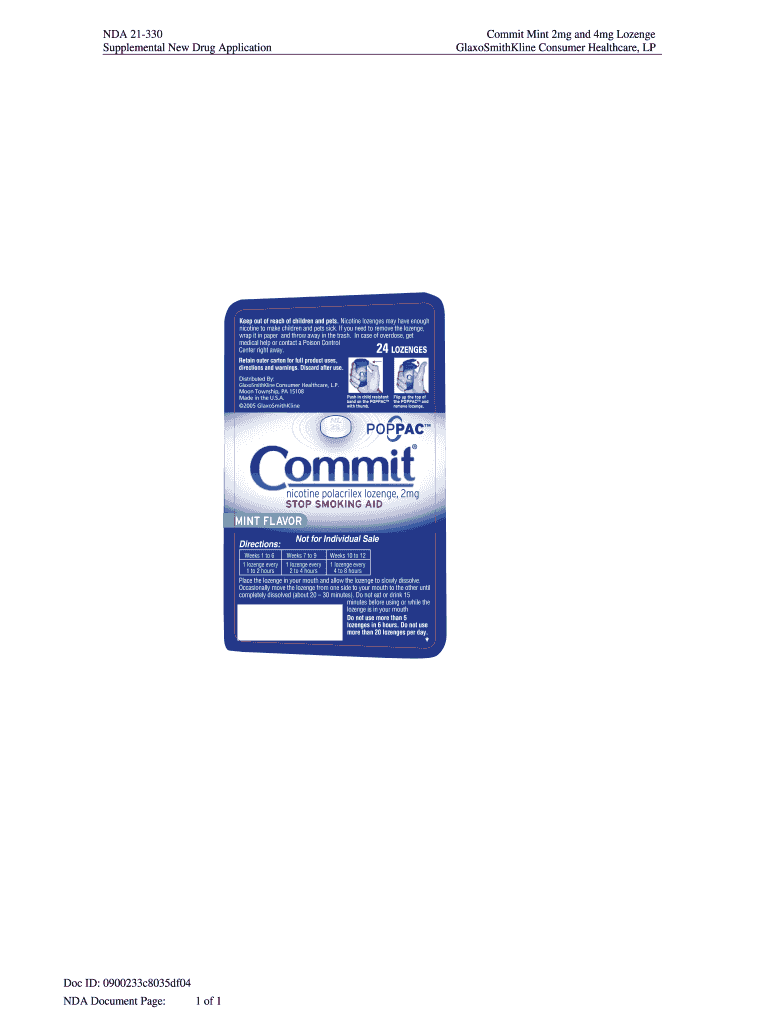
Supplemental New Drug ApplicationCommit Mint 2 mg and 4 mg Lozenge
GlaxoSmithKline Consumer Healthcare, Upkeep out of reach of children and pets. Nicotine lozenges may have enough
nicotine
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign supplemental new drug application

Edit your supplemental new drug application form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your supplemental new drug application form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit supplemental new drug application online
To use the professional PDF editor, follow these steps below:
1
Log in to your account. Click on Start Free Trial and register a profile if you don't have one.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit supplemental new drug application. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
pdfFiller makes dealing with documents a breeze. Create an account to find out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out supplemental new drug application

How to fill out supplemental new drug application
01
To fill out a supplemental new drug application, follow these steps:
02
Collect all necessary information and documents related to the new drug application.
03
Review the original new drug application to understand the purpose and scope of the supplement.
04
Determine the specific sections or areas that require supplementation.
05
Prepare the supplemental information, including updated data, clinical trial results, or additional studies.
06
Fill out the specific sections of the application form that pertain to the supplement.
07
Provide a detailed explanation of the changes or additions being made in the supplement.
08
Include any supporting documents or references that validate the supplement.
09
Review the completed application for accuracy and completeness.
10
Submit the supplemental new drug application to the appropriate regulatory authority.
11
Follow up and respond to any additional requests or inquiries from the regulatory authority.
Who needs supplemental new drug application?
01
A supplemental new drug application is needed by pharmaceutical companies or sponsors who have already received approval for a new drug application (NDA) and need to make modifications, updates, or expansions to the approved product.
02
Specific cases may include:
03
- Changes to the drug's formulation, dosage strength, or administration route
04
- Additions of new indications or populations
05
- Modifications based on new clinical trial findings or safety information
06
- Labeling updates or changes
07
- Manufacturing process changes
08
- Post-approval commitments or obligations
09
- Revisions to previously approved drug components or characteristics
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I manage my supplemental new drug application directly from Gmail?
Using pdfFiller's Gmail add-on, you can edit, fill out, and sign your supplemental new drug application and other papers directly in your email. You may get it through Google Workspace Marketplace. Make better use of your time by handling your papers and eSignatures.
Where do I find supplemental new drug application?
The premium subscription for pdfFiller provides you with access to an extensive library of fillable forms (over 25M fillable templates) that you can download, fill out, print, and sign. You won’t have any trouble finding state-specific supplemental new drug application and other forms in the library. Find the template you need and customize it using advanced editing functionalities.
Can I edit supplemental new drug application on an Android device?
The pdfFiller app for Android allows you to edit PDF files like supplemental new drug application. Mobile document editing, signing, and sending. Install the app to ease document management anywhere.
What is supplemental new drug application?
Supplemental New Drug Application (sNDA) is an application submitted to the FDA for a significant change in the safety, efficacy, or use of a drug product.
Who is required to file supplemental new drug application?
Any pharmaceutical company that holds an approved new drug application (NDA) or abbreviated new drug application (ANDA) may be required to file a supplemental new drug application.
How to fill out supplemental new drug application?
To fill out a supplemental new drug application, the company must provide detailed information about the proposed changes, including data supporting the changes, along with any other relevant documentation.
What is the purpose of supplemental new drug application?
The purpose of a supplemental new drug application is to request approval for a significant change in the safety, efficacy, or use of a drug product that was not included in the original NDA or ANDA.
What information must be reported on supplemental new drug application?
The supplemental new drug application must include detailed information about the proposed changes, supporting data, any new safety or efficacy information, and any other relevant documentation.
Fill out your supplemental new drug application online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Supplemental New Drug Application is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.




















