Get the free Drug Audit Checklist 23
Show details
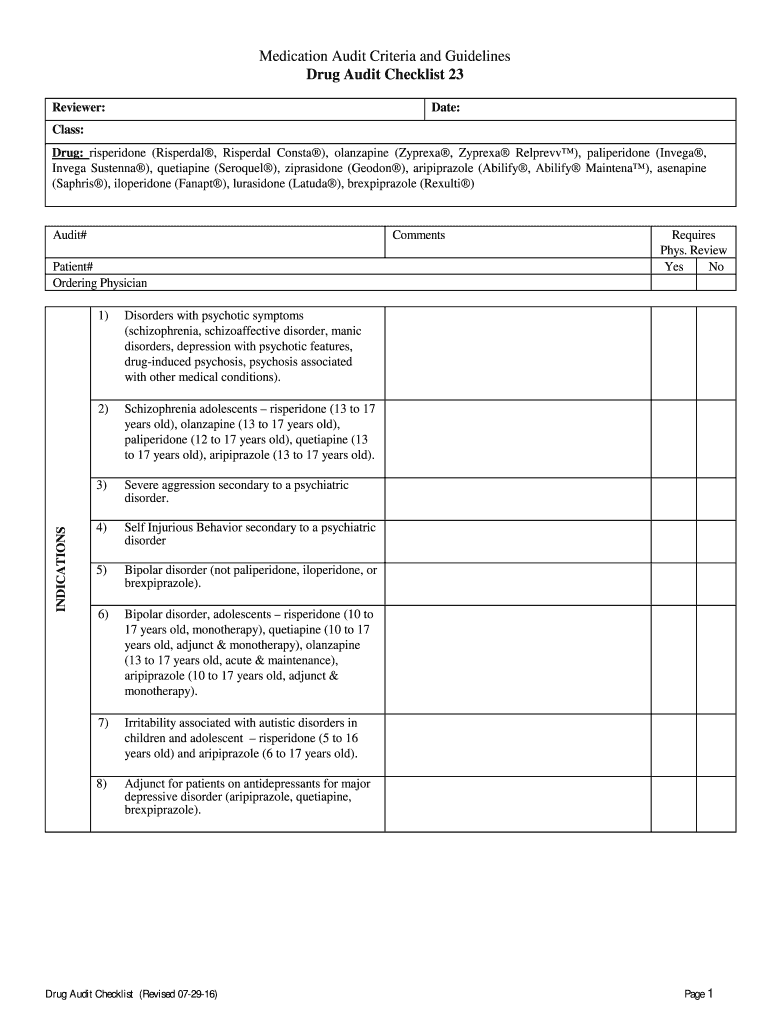
Medication Audit Criteria and Guidelines
Drug Audit Checklist 23
Reviewer:Date:Class:
Drug: risperidone (Risperdal, Risperdal Cons ta), olanzapine (Zyprexa, Zyprexa Relieve), paliperidone (Invest,
Invest
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign drug audit checklist 23

Edit your drug audit checklist 23 form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your drug audit checklist 23 form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit drug audit checklist 23 online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Upload a document. Select Add New on your Dashboard and transfer a file into the system in one of the following ways: by uploading it from your device or importing from the cloud, web, or internal mail. Then, click Start editing.
3
Edit drug audit checklist 23. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Save your file. Select it from your list of records. Then, move your cursor to the right toolbar and choose one of the exporting options. You can save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud, among other things.
With pdfFiller, it's always easy to work with documents.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out drug audit checklist 23

How to fill out drug audit checklist 23
01
To fill out drug audit checklist 23, follow these steps:
02
Begin by reviewing the checklist and familiarizing yourself with the items that need to be assessed.
03
Start by gathering all the relevant information and documents related to the drug audit.
04
Go through each point in the checklist and evaluate whether it is compliant or not.
05
If there are any discrepancies or non-compliant items, make a note of them and provide additional details if required.
06
Ensure that all the necessary information and supporting documents are accurately filled out and attached to the checklist.
07
Double-check for any errors or omissions before submitting the completed checklist for review.
08
If applicable, provide any additional comments or explanations for specific items in the checklist.
09
Finally, submit the completed drug audit checklist 23 to the designated authority for further evaluation and action.
Who needs drug audit checklist 23?
01
Drug audit checklist 23 is typically needed by organizations involved in pharmaceutical manufacturing, distribution, or healthcare facilities.
02
It is useful for pharmaceutical companies, hospitals, pharmacies, and other entities to ensure compliance with regulatory requirements and maintain quality control in drug-related processes.
03
Individuals responsible for conducting regular audits and inspections, quality assurance personnel, and regulatory authorities may also require drug audit checklist 23 to assess and verify compliance.
Fill
form
: Try Risk Free






People Also Ask about
What is GMP audit checklist?
A Good Manufacturing Practices (GMP) audit checklist is a tool used by manufacturers to ensure that food, pharmaceutical, medical, and cosmetic products are of consistent quality and in compliance with manufacturing standards.
What is pharmacy audit?
A pharmacy audit is a formal review of operations and processes to make sure that pharmacies are compliant with pharmacy regulations and other related agreements. The pharmacy audit process can come in many different types, including desktop, onsite, prepay claims review, and investigational pharmacy audits.
How do you prepare a quality audit checklist?
Below are the phases of using a quality audit checklist for quality audits: Identify audit objectives. Determine audit scope. Conduct the audit. Report audit findings. Provide corrective action. Check progress of corrective action. Determine if gaps have been addressed. Update status of compliance.
What is included in an audit checklist?
An audit checklist may be a document or tool that to facilitate an audit programme which contains documented information such as the scope of the audit, evidence collection, audit tests and methods, analysis of the results as well as the conclusion and follow up actions such as corrective and preventive actions.
How do you complete a pharmacy audit?
Pharmacy Audit Checklist Evaluate current prescribing practices, controlled substance management, invoice management, and billing practices. Take/attach photos of best practices or non-compliance with HCFAC. Assign corrective actions in real-time. Complete the pharmacy audit with a digital signature.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I send drug audit checklist 23 for eSignature?
When your drug audit checklist 23 is finished, send it to recipients securely and gather eSignatures with pdfFiller. You may email, text, fax, mail, or notarize a PDF straight from your account. Create an account today to test it.
How do I complete drug audit checklist 23 online?
pdfFiller has made filling out and eSigning drug audit checklist 23 easy. The solution is equipped with a set of features that enable you to edit and rearrange PDF content, add fillable fields, and eSign the document. Start a free trial to explore all the capabilities of pdfFiller, the ultimate document editing solution.
How can I edit drug audit checklist 23 on a smartphone?
You may do so effortlessly with pdfFiller's iOS and Android apps, which are available in the Apple Store and Google Play Store, respectively. You may also obtain the program from our website: https://edit-pdf-ios-android.pdffiller.com/. Open the application, sign in, and begin editing drug audit checklist 23 right away.
What is drug audit checklist 23?
Drug audit checklist 23 is a document used to track and verify the inventory of drugs in a pharmacy or healthcare facility.
Who is required to file drug audit checklist 23?
All pharmacies and healthcare facilities that dispense drugs are required to file drug audit checklist 23.
How to fill out drug audit checklist 23?
Drug audit checklist 23 should be filled out by recording the quantity of each drug in inventory, verifying expiration dates, and documenting any discrepancies.
What is the purpose of drug audit checklist 23?
The purpose of drug audit checklist 23 is to ensure accurate tracking of drug inventory, detect any potential issues or discrepancies, and maintain compliance with regulations.
What information must be reported on drug audit checklist 23?
Information such as drug name, quantity in stock, expiration date, lot number, and any discrepancies found during the audit must be reported on drug audit checklist 23.
Fill out your drug audit checklist 23 online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Drug Audit Checklist 23 is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.
























