Get the free Nomenclature #1: Binary Ionic Compounds
Show details
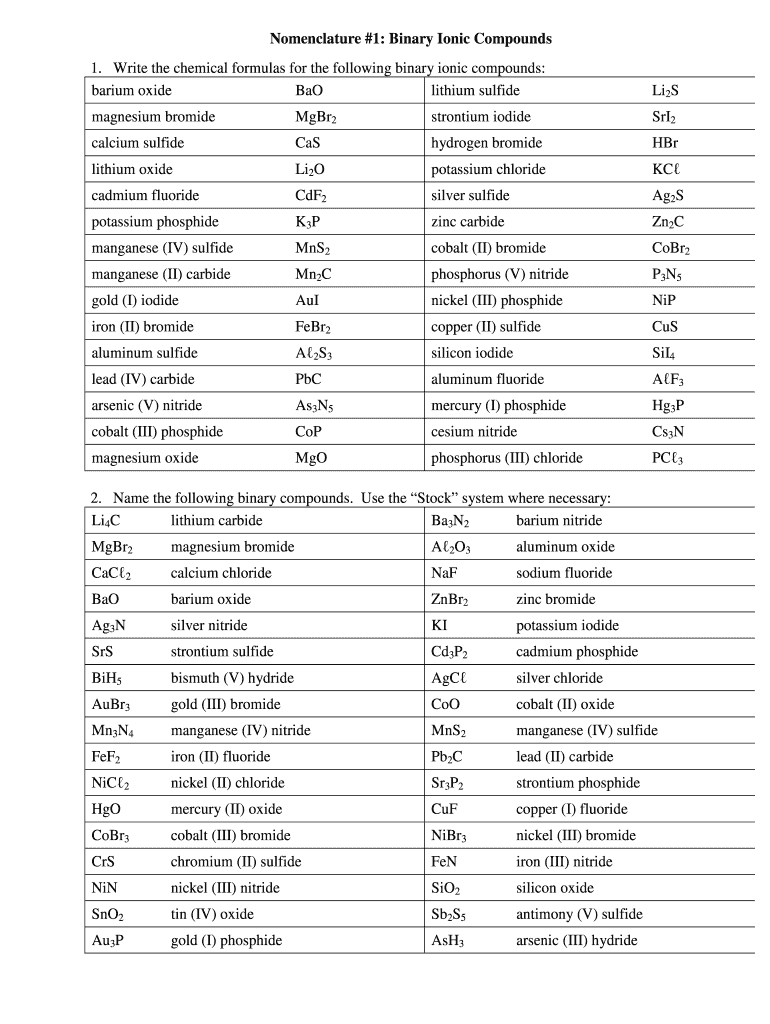
Nomenclature #1: Binary Ionic Compounds
1. Write the chemical formulas for the following binary ionic compounds:
barium oxide
Bad
lithium sulfideLi2Smagnesium bromideMgBr2strontium iodideSrI2calcium
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign nomenclature 1 binary ionic

Edit your nomenclature 1 binary ionic form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your nomenclature 1 binary ionic form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit nomenclature 1 binary ionic online
To use the professional PDF editor, follow these steps below:
1
Create an account. Begin by choosing Start Free Trial and, if you are a new user, establish a profile.
2
Upload a document. Select Add New on your Dashboard and transfer a file into the system in one of the following ways: by uploading it from your device or importing from the cloud, web, or internal mail. Then, click Start editing.
3
Edit nomenclature 1 binary ionic. Replace text, adding objects, rearranging pages, and more. Then select the Documents tab to combine, divide, lock or unlock the file.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
With pdfFiller, it's always easy to work with documents.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out nomenclature 1 binary ionic

How to Fill Out Nomenclature 1 Binary Ionic:
01
Start by identifying the cation and anion in the compound. The cation is typically a metal, while the anion is a non-metal.
02
Write the cation name first, followed by the anion name. If the cation has more than one possible charge, indicate the charge using Roman numerals in parentheses. For example, Fe(II) or Fe(III) for iron.
03
If the anion is a single element, simply write its name with an -ide ending. For example, chlorine becomes chloride.
04
If the anion is a polyatomic ion, write its name as it is. Do not change the ending or modify the name.
05
Check if any subscripts are needed. The subscripts indicate the number of each ion needed to balance the charges. If the charges on the ions are the same, no subscripts are needed. If the charges are different, use subscripts to balance them. For example, if you have calcium with a 2+ charge and chlorine with a 1- charge, you will need to write CaCl2.
06
Finally, make sure your compound is neutral. If necessary, adjust the subscripts to ensure that the positive and negative charges balance.
Who needs nomenclature 1 binary ionic?
01
Chemistry students: Nomenclature 1 binary ionic is commonly taught in chemistry courses, so students studying this subject need to understand how to fill out this nomenclature.
02
Scientists and researchers: Nomenclature 1 binary ionic is a fundamental concept in chemistry and is used in scientific research and analysis. Scientists and researchers working in various fields like materials science, pharmaceuticals, and environmental science need to be familiar with this nomenclature.
03
Professionals in related industries: Professionals working in industries such as chemical manufacturing, quality control, and analytical chemistry also require knowledge of nomenclature 1 binary ionic. They may need to identify and communicate chemical compounds accurately in their work.
04
Educators: Chemistry teachers and educators need to know how to teach nomenclature 1 binary ionic effectively to their students. They need to be well-versed in this topic to help students learn and apply this naming system correctly.
05
Anyone interested in chemistry: Even individuals with a general interest in chemistry can benefit from understanding nomenclature 1 binary ionic. It allows them to understand and appreciate the names and formulas of common compounds they encounter in their daily lives, such as table salt (sodium chloride) or baking soda (sodium bicarbonate).
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I send nomenclature 1 binary ionic for eSignature?
To distribute your nomenclature 1 binary ionic, simply send it to others and receive the eSigned document back instantly. Post or email a PDF that you've notarized online. Doing so requires never leaving your account.
Where do I find nomenclature 1 binary ionic?
The premium version of pdfFiller gives you access to a huge library of fillable forms (more than 25 million fillable templates). You can download, fill out, print, and sign them all. State-specific nomenclature 1 binary ionic and other forms will be easy to find in the library. Find the template you need and use advanced editing tools to make it your own.
How do I make edits in nomenclature 1 binary ionic without leaving Chrome?
Download and install the pdfFiller Google Chrome Extension to your browser to edit, fill out, and eSign your nomenclature 1 binary ionic, which you can open in the editor with a single click from a Google search page. Fillable documents may be executed from any internet-connected device without leaving Chrome.
What is nomenclature 1 binary ionic?
Nomenclature 1 binary ionic is a naming system for compounds that consist of a cation and an anion.
Who is required to file nomenclature 1 binary ionic?
Chemists and students studying chemistry are required to use nomenclature 1 binary ionic.
How to fill out nomenclature 1 binary ionic?
Nomenclature 1 binary ionic is filled out by identifying the cation and anion in a compound and naming them accordingly.
What is the purpose of nomenclature 1 binary ionic?
The purpose of nomenclature 1 binary ionic is to provide a standardized way of naming ionic compounds.
What information must be reported on nomenclature 1 binary ionic?
Nomenclature 1 binary ionic must report the name of the cation and anion in an ionic compound.
Fill out your nomenclature 1 binary ionic online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Nomenclature 1 Binary Ionic is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.


















