Get the free FDA & CDC: Collaboration & Challenges FSMA, Food Outbreaks ... - fdli
Show details
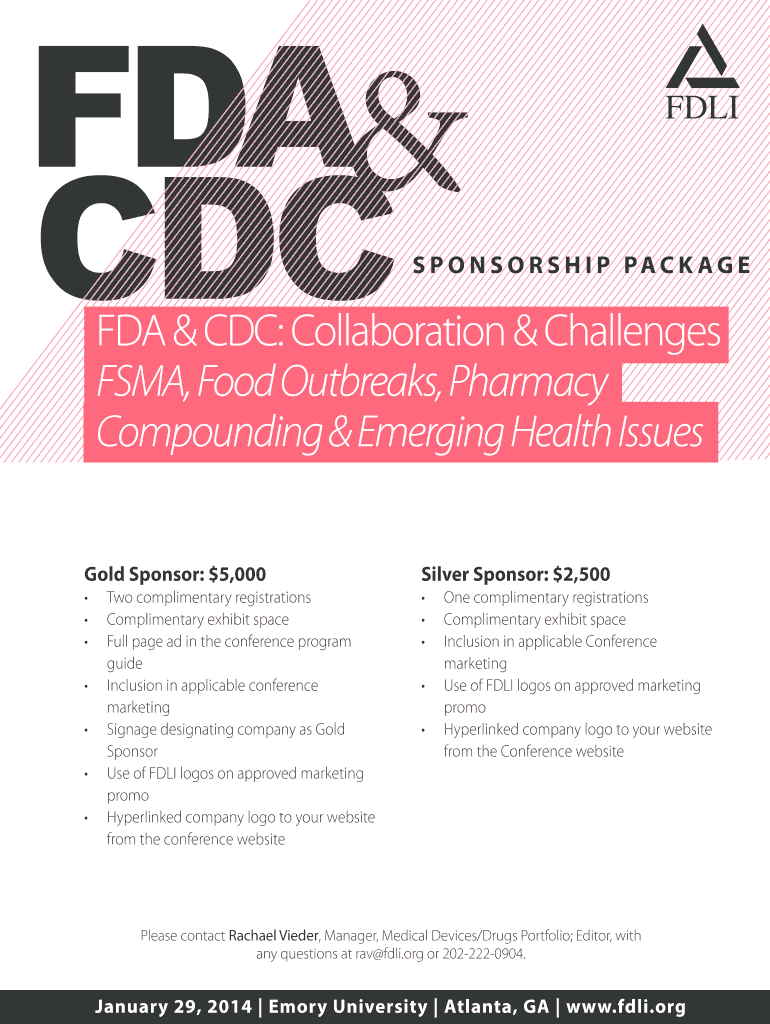
FDA CDC S P O N S O R S H I P PAC K AG E FDA & CDC: Collaboration & Challenges FSMA, Food Outbreaks, Pharmacy Compounding & Emerging Health Issues Gold Sponsor: $5,000 Silver Sponsor: $2,500 Two complimentary
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign fda amp cdc collaboration

Edit your fda amp cdc collaboration form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your fda amp cdc collaboration form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit fda amp cdc collaboration online
To use the professional PDF editor, follow these steps:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit fda amp cdc collaboration. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Get your file. When you find your file in the docs list, click on its name and choose how you want to save it. To get the PDF, you can save it, send an email with it, or move it to the cloud.
pdfFiller makes dealing with documents a breeze. Create an account to find out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out fda amp cdc collaboration

How to fill out FDA amp CDC collaboration:
01
Understand the purpose: Before starting to fill out the FDA amp CDC collaboration, it is important to understand why this collaboration is needed. The partnership between the FDA (Food and Drug Administration) and CDC (Centers for Disease Control and Prevention) aims to enhance public health by improving the safety and efficacy of medical products and responding to public health emergencies.
02
Review the collaboration requirements: Familiarize yourself with the specific requirements and guidelines set forth by both the FDA and CDC for this collaboration. This may involve reading through collaboration agreements, policy documents, and any additional resources provided by both agencies.
03
Establish clear communication channels: It is crucial to establish effective communication channels between the FDA and CDC. This can include regular meetings, conference calls, email correspondence, and potentially even in-person discussions. Clear lines of communication are essential for ensuring a smooth collaboration process.
04
Define roles and responsibilities: Clearly identify the roles and responsibilities of each participating entity. Understand the specific functions, tasks, and deliverables that are expected from both the FDA and CDC. This will help in avoiding any confusion or overlapping efforts during the collaboration.
05
Gather relevant information and data: Collect any necessary information or data required for the collaboration. This may include research studies, clinical trial results, population health data, or any other relevant resources. Having access to accurate and up-to-date information is crucial for effective decision-making and problem-solving during the collaboration.
06
Collaborate on joint initiatives: Work together with the FDA and CDC to identify and prioritize joint initiatives. This can involve sharing expertise, resources, and knowledge to tackle public health challenges. Collaborate on projects such as drug safety monitoring, disease surveillance, outbreak response, and sharing best practices.
07
Monitor and evaluate progress: Regularly monitor and evaluate the progress of the FDA amp CDC collaboration. This can involve assessing the outcomes, impact, and overall effectiveness of joint initiatives. Identifying areas of improvement and adjusting strategies accordingly will help enhance future collaborations.
Who needs FDA amp CDC collaboration:
01
Researchers and scientists: Collaboration between the FDA and CDC is crucial for researchers and scientists working in public health, medical product development, and epidemiology. It provides them with access to valuable data, resources, and expertise, enabling them to conduct impactful research and contribute to the improvement of public health outcomes.
02
Healthcare professionals: The collaboration between the FDA and CDC has a direct impact on healthcare professionals, especially in areas such as drug safety, disease surveillance, and outbreak response. Collaboration efforts facilitate the delivery of safe and effective medical products to patients, as well as the identification and prevention of public health threats.
03
Regulatory agencies: Regulatory agencies responsible for overseeing the safety and efficacy of medical products also benefit from FDA amp CDC collaboration. Joining forces allows them to pool resources, expertise, and knowledge, leading to more efficient and comprehensive regulatory processes.
04
Government entities: Collaboration between the FDA and CDC is vital for government entities responsible for public health, emergency response, and policy-making. By working together, they can leverage each other's strengths and resources to develop and implement evidence-based interventions, policies, and guidelines.
05
General public: Ultimately, the FDA amp CDC collaboration serves the general public by striving to improve public health outcomes and enhance medical product safety. By conducting joint research, sharing information, and coordinating responses to public health emergencies, this collaboration aims to protect and promote the well-being of all individuals.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I fill out the fda amp cdc collaboration form on my smartphone?
You can easily create and fill out legal forms with the help of the pdfFiller mobile app. Complete and sign fda amp cdc collaboration and other documents on your mobile device using the application. Visit pdfFiller’s webpage to learn more about the functionalities of the PDF editor.
How do I edit fda amp cdc collaboration on an iOS device?
You can. Using the pdfFiller iOS app, you can edit, distribute, and sign fda amp cdc collaboration. Install it in seconds at the Apple Store. The app is free, but you must register to buy a subscription or start a free trial.
How do I fill out fda amp cdc collaboration on an Android device?
Complete your fda amp cdc collaboration and other papers on your Android device by using the pdfFiller mobile app. The program includes all of the necessary document management tools, such as editing content, eSigning, annotating, sharing files, and so on. You will be able to view your papers at any time as long as you have an internet connection.
What is fda amp cdc collaboration?
FDA and CDC collaboration refers to the partnership between the Food and Drug Administration and the Centers for Disease Control and Prevention to coordinate efforts in regulating food safety and public health.
Who is required to file fda amp cdc collaboration?
Companies and organizations involved in the production, distribution, and monitoring of food and drug products are required to file FDA and CDC collaboration reports.
How to fill out fda amp cdc collaboration?
The FDA and CDC collaboration reports can be filled out online through the official websites of the respective agencies or through designated electronic submission portals.
What is the purpose of fda amp cdc collaboration?
The purpose of FDA and CDC collaboration is to ensure the safety and efficacy of food and drug products, as well as to monitor and respond to public health concerns and outbreaks.
What information must be reported on fda amp cdc collaboration?
The FDA and CDC collaboration reports typically require information on product testing, safety data, adverse events, recalls, and any other relevant information related to food and drug products.
Fill out your fda amp cdc collaboration online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Fda Amp Cdc Collaboration is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.



















