Get the free Autoclave Quality Control Measures - iacuc ucsf
Show details
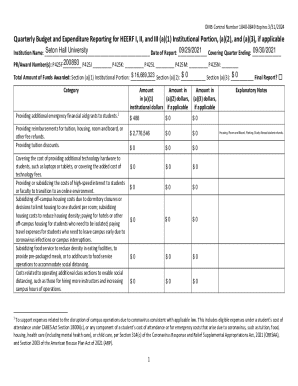
Autoclave Quality Control Measures
IACUC Guidelines
Effective Date: May 2016Guidelines for Autoclave Quality Control Measures
Autoclaves offer a reliable and effective way to sterilize instruments.
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign autoclave quality control measures

Edit your autoclave quality control measures form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your autoclave quality control measures form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing autoclave quality control measures online
Follow the steps below to benefit from a competent PDF editor:
1
Log in to account. Start Free Trial and register a profile if you don't have one.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit autoclave quality control measures. Rearrange and rotate pages, add new and changed texts, add new objects, and use other useful tools. When you're done, click Done. You can use the Documents tab to merge, split, lock, or unlock your files.
4
Get your file. When you find your file in the docs list, click on its name and choose how you want to save it. To get the PDF, you can save it, send an email with it, or move it to the cloud.
pdfFiller makes working with documents easier than you could ever imagine. Register for an account and see for yourself!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out autoclave quality control measures

How to fill out autoclave quality control measures
01
Step 1: Start by familiarizing yourself with the autoclave equipment and its operating instructions.
02
Step 2: Ensure that all necessary supplies and tools are readily available, including sterilization bags, indicators, and record-keeping forms.
03
Step 3: Pre-clean the items to be autoclaved by removing visible debris or organic material.
04
Step 4: Place the items inside the sterilization bags, ensuring that they are not overcrowded.
05
Step 5: Seal the bags securely, leaving a small vent to allow for proper steam penetration.
06
Step 6: Place indicator strips or tapes on the bags to verify whether proper sterilization conditions have been met.
07
Step 7: Load the autoclave with the prepared bags, following the manufacturer's instructions for maximum load capacity and placement.
08
Step 8: Set the appropriate sterilization cycle and parameters, such as temperature and time, based on the type of items being sterilized.
09
Step 9: Start the autoclave cycle and monitor its progress to ensure proper sterilization.
10
Step 10: Once the cycle is complete, allow the autoclave to depressurize before opening the door.
11
Step 11: Carefully remove the sterilized items from the autoclave and inspect them for any signs of damage or improper sterilization.
12
Step 12: Record all relevant information, including the date, time, cycle parameters, and the person responsible for the autoclave process.
13
Step 13: Store the sterilized items in a clean, designated area until they are ready for use.
Who needs autoclave quality control measures?
01
Autoclave quality control measures are essential for any facility or organization that requires sterilization of equipment or materials.
02
This includes but is not limited to healthcare facilities, research laboratories, manufacturing plants, veterinary clinics, and tattoo parlors.
03
Ensuring proper autoclave quality control helps prevent the spread of infectious diseases, maintains equipment functionality, and ensures the safety of both patients and staff.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I modify autoclave quality control measures without leaving Google Drive?
Simplify your document workflows and create fillable forms right in Google Drive by integrating pdfFiller with Google Docs. The integration will allow you to create, modify, and eSign documents, including autoclave quality control measures, without leaving Google Drive. Add pdfFiller’s functionalities to Google Drive and manage your paperwork more efficiently on any internet-connected device.
How can I send autoclave quality control measures to be eSigned by others?
To distribute your autoclave quality control measures, simply send it to others and receive the eSigned document back instantly. Post or email a PDF that you've notarized online. Doing so requires never leaving your account.
How do I make edits in autoclave quality control measures without leaving Chrome?
Adding the pdfFiller Google Chrome Extension to your web browser will allow you to start editing autoclave quality control measures and other documents right away when you search for them on a Google page. People who use Chrome can use the service to make changes to their files while they are on the Chrome browser. pdfFiller lets you make fillable documents and make changes to existing PDFs from any internet-connected device.
What is autoclave quality control measures?
Autoclave quality control measures are procedures put in place to ensure that autoclaves are functioning properly and effectively sterilizing equipment.
Who is required to file autoclave quality control measures?
Healthcare facilities and laboratories that use autoclaves are typically required to file autoclave quality control measures.
How to fill out autoclave quality control measures?
Autoclave quality control measures are usually filled out by documenting daily, weekly, and monthly checks on autoclave performance and maintenance.
What is the purpose of autoclave quality control measures?
The purpose of autoclave quality control measures is to maintain the effectiveness and safety of autoclaves in sterilizing equipment.
What information must be reported on autoclave quality control measures?
Information such as autoclave temperature, pressure, cycle duration, and maintenance records must be reported on autoclave quality control measures.
Fill out your autoclave quality control measures online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Autoclave Quality Control Measures is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.