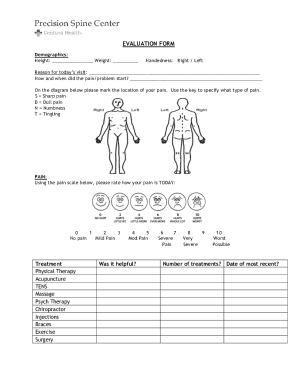
Get the free Clinical Trial Management
Show details
Clinical Trial Management UOB RMS reference number: UoBCLNSOP001Purpose: The purpose of this procedure is to explain how clinical trials should be conducted within the University of Birmingham (UOB).
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign clinical trial management

Edit your clinical trial management form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your clinical trial management form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing clinical trial management online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Check your account. If you don't have a profile yet, click Start Free Trial and sign up for one.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit clinical trial management. Rearrange and rotate pages, insert new and alter existing texts, add new objects, and take advantage of other helpful tools. Click Done to apply changes and return to your Dashboard. Go to the Documents tab to access merging, splitting, locking, or unlocking functions.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
pdfFiller makes dealing with documents a breeze. Create an account to find out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out clinical trial management

How to Fill Out Clinical Trial Management:
01
Understand the purpose: Before filling out any clinical trial management documents, it is crucial to have a clear understanding of the purpose of the clinical trial. This includes identifying the research question or objective, determining the target population, and defining the outcomes or measures to be assessed.
02
Gather necessary information: Collect all relevant information needed to complete the clinical trial management documents. This may include details about the study protocol, the investigational product, study sites, and any regulatory requirements or guidelines that need to be followed.
03
Start with the basics: Begin by filling out the basic information, such as the trial title, trial number, and principal investigator's name. These details help identify the specific trial being conducted and the responsible individuals.
04
Develop the trial protocol: Write a comprehensive trial protocol that outlines the objectives, study design, methodology, eligibility criteria, interventions, assessments, and any other relevant information. This protocol serves as a blueprint for the entire trial and should be based on scientific and ethical principles.
05
Design the case report forms (CRFs): Create CRFs tailored to collect specific data points for the study. These forms will be used to collect and record data from participants throughout the trial. Ensure the CRFs are clear, concise, and aligned with the trial objectives.
06
Consider regulatory requirements: Depending on the location and nature of the trial, there may be specific regulatory requirements that need to be fulfilled. Familiarize yourself with these regulations and fill out any necessary documentation accordingly. This might include obtaining approval from ethics committees or regulatory agencies.
07
Collaborate with investigators and site staff: Work closely with investigators and site staff to ensure that they understand the process of filling out clinical trial management documents. Provide clear instructions and guidance on how to accurately complete the forms, and be available to address any questions or concerns they may have.
08
Implement quality control measures: Establish quality control measures to ensure the accuracy, completeness, and consistency of the data collected in the trial. This may involve periodic data monitoring, site visits, and data validation procedures.
Who needs clinical trial management?
01
Pharmaceutical companies and biotech firms: These organizations are primarily responsible for initiating and conducting clinical trials as part of their drug development process. Clinical trial management is crucial for ensuring the successful execution and monitoring of trials.
02
Contract research organizations (CROs): CROs are hired by pharmaceutical companies or other sponsors to manage various aspects of clinical trials. They play a vital role in coordinating and overseeing the trial process, including documentation and data management.
03
Academic research institutions: Academic institutions often conduct clinical trials as part of their research programs. Clinical trial management helps academic researchers streamline their trial processes, meet regulatory requirements, and ensure data integrity.
04
Regulatory authorities: Regulatory bodies, such as the Food and Drug Administration (FDA) in the United States or the European Medicines Agency (EMA) in Europe, require clinical trial management to review and evaluate research data before approving new drugs or treatments.
05
Ethics committees: Clinical trial management supports the work of ethics committees, ensuring that trials are conducted ethically, with proper informed consent, and the safety and welfare of participants are safeguarded.
In summary, clinical trial management involves effectively filling out trial documentation, creating protocols, designing CRFs, adhering to regulatory requirements, and collaborating with investigators and site staff. It is essential for organizations involved in drug development, contract research organizations, academic institutions, regulatory bodies, and ethics committees.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I modify my clinical trial management in Gmail?
It's easy to use pdfFiller's Gmail add-on to make and edit your clinical trial management and any other documents you get right in your email. You can also eSign them. Take a look at the Google Workspace Marketplace and get pdfFiller for Gmail. Get rid of the time-consuming steps and easily manage your documents and eSignatures with the help of an app.
How do I edit clinical trial management straight from my smartphone?
The pdfFiller mobile applications for iOS and Android are the easiest way to edit documents on the go. You may get them from the Apple Store and Google Play. More info about the applications here. Install and log in to edit clinical trial management.
How do I fill out the clinical trial management form on my smartphone?
The pdfFiller mobile app makes it simple to design and fill out legal paperwork. Complete and sign clinical trial management and other papers using the app. Visit pdfFiller's website to learn more about the PDF editor's features.
What is clinical trial management?
Clinical trial management is the process of planning, organizing, and overseeing the various aspects of a clinical trial to ensure it is conducted in compliance with regulatory requirements and protocols.
Who is required to file clinical trial management?
The sponsor or principal investigator of the clinical trial is typically responsible for filing the clinical trial management.
How to fill out clinical trial management?
Clinical trial management is typically filled out using a designated software system or data management platform that captures all the necessary information related to the trial.
What is the purpose of clinical trial management?
The purpose of clinical trial management is to ensure the successful planning, execution, and monitoring of a clinical trial to generate reliable and valid data.
What information must be reported on clinical trial management?
Clinical trial management must include details on the study protocol, participant demographics, adverse events, protocol deviations, and study outcomes.
Fill out your clinical trial management online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Clinical Trial Management is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.