Get the free RE: COMMISSION ON HUMAN
Show details
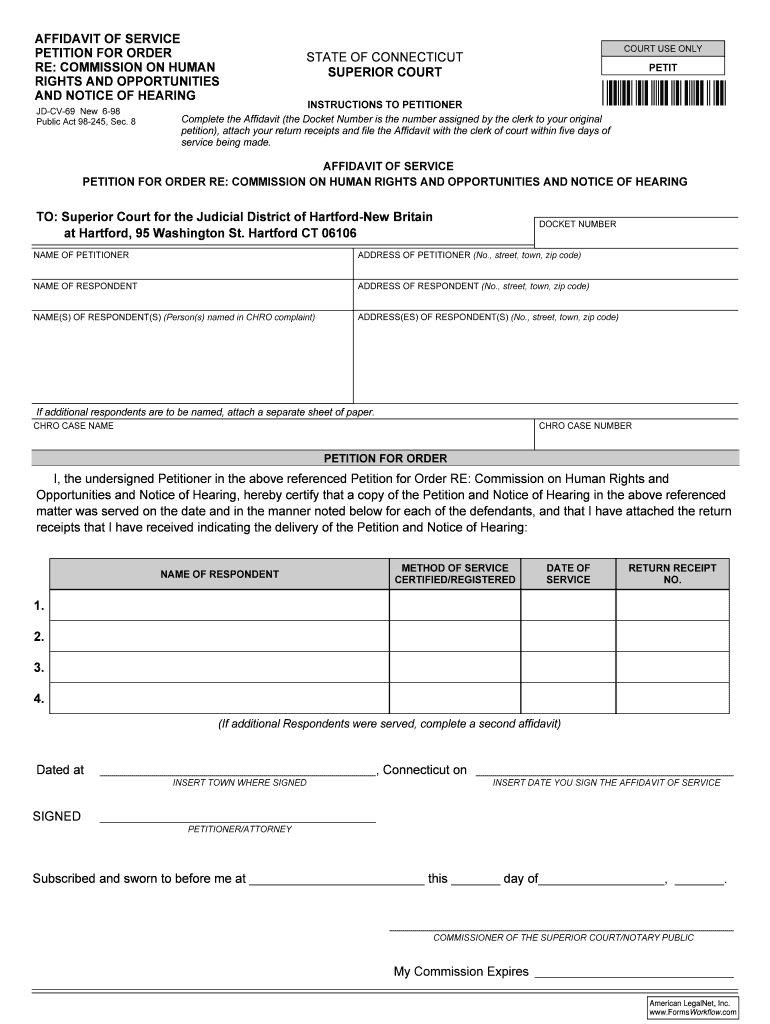
AFFIDAVIT OF SERVICE
PETITION FOR ORDER
RE: COMMISSION ON HUMAN
RIGHTS AND OPPORTUNITIES
AND NOTICE OF HEARING
JDCV69 New 698
Public Act 98245, Sec. 8COURT USE ONSTAGE OF CONNECTICUT
SUPERIOR COURTPETITINSTRUCTIONS
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign re commission on human

Edit your re commission on human form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your re commission on human form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing re commission on human online
To use the services of a skilled PDF editor, follow these steps below:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit re commission on human. Replace text, adding objects, rearranging pages, and more. Then select the Documents tab to combine, divide, lock or unlock the file.
4
Save your file. Select it from your list of records. Then, move your cursor to the right toolbar and choose one of the exporting options. You can save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud, among other things.
With pdfFiller, dealing with documents is always straightforward.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out re commission on human

How to fill out re commission on human
01
To fill out a re commission on human form, follow these steps:
02
Start by reading the instructions on the form carefully to understand the requirements and any specific guidelines.
03
Make sure you have all the necessary information and documentation required to complete the form.
04
Begin by providing your personal details, such as your name, contact information, and any identification numbers or references.
05
Next, it is important to thoroughly describe the reason for the re commission on human. Provide a detailed explanation and include any relevant supporting evidence or documentation if required.
06
Double-check all the information you have provided to ensure accuracy and completeness.
07
Review any additional sections or questions on the form and provide the requested information accordingly.
08
If there are any declarations or statements that need to be signed, make sure to read them carefully, understand their implications, and sign as required.
09
Make a copy of the completed form and any supporting documentation for your records.
10
Submit the filled-out re commission on human form by the designated method, whether it is through mail, online submission, or in-person delivery.
11
If applicable, ensure that any required fees or payments are included with the submission.
12
Wait for confirmation or further instructions regarding the re commission on human process, which may vary depending on the specific circumstances.
Who needs re commission on human?
01
Re commission on human forms may be required by various individuals or entities, including:
02
- Employees who need to request a re commission on their employment status or benefits
03
- Employers who need to submit re commission on human forms for their employees
04
- Government agencies or departments responsible for managing human resources or labor regulations
05
- Individuals seeking legal or administrative remedies related to human rights or labor issues
06
- Organizations or entities involved in human resources management or personnel administration
07
- Individuals involved in contractual agreements or disputes related to human resources or employment
08
- Any person or entity involved in matters concerning the evaluation or re commission on human of an individual or group.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I make changes in re commission on human?
The editing procedure is simple with pdfFiller. Open your re commission on human in the editor. You may also add photos, draw arrows and lines, insert sticky notes and text boxes, and more.
Can I create an electronic signature for the re commission on human in Chrome?
You certainly can. You get not just a feature-rich PDF editor and fillable form builder with pdfFiller, but also a robust e-signature solution that you can add right to your Chrome browser. You may use our addon to produce a legally enforceable eSignature by typing, sketching, or photographing your signature with your webcam. Choose your preferred method and eSign your re commission on human in minutes.
How do I edit re commission on human straight from my smartphone?
Using pdfFiller's mobile-native applications for iOS and Android is the simplest method to edit documents on a mobile device. You may get them from the Apple App Store and Google Play, respectively. More information on the apps may be found here. Install the program and log in to begin editing re commission on human.
What is re commission on human?
Re commission on human is a process of reconsidering, reviewing, or evaluating the suitability of an individual to continue serving as a human subject in research studies or clinical trials.
Who is required to file re commission on human?
Researchers, institutions, or organizations conducting research studies or clinical trials involving human subjects are typically required to file re commission on human.
How to fill out re commission on human?
To fill out re commission on human, researchers need to provide updated information about the research study or clinical trial, informed consent procedures, risk assessment, and any changes in the protocol involving human subjects.
What is the purpose of re commission on human?
The purpose of re commission on human is to ensure the protection of human subjects participating in research studies or clinical trials, and to review the ethical considerations and scientific validity of the study.
What information must be reported on re commission on human?
Information such as updates on the research study or clinical trial protocol, informed consent procedures, risk assessment, participant recruitment methods, and any adverse events related to human subjects must be reported on re commission on human.
Fill out your re commission on human online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Re Commission On Human is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















