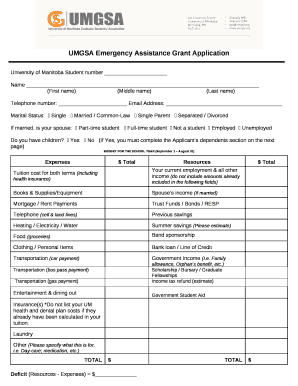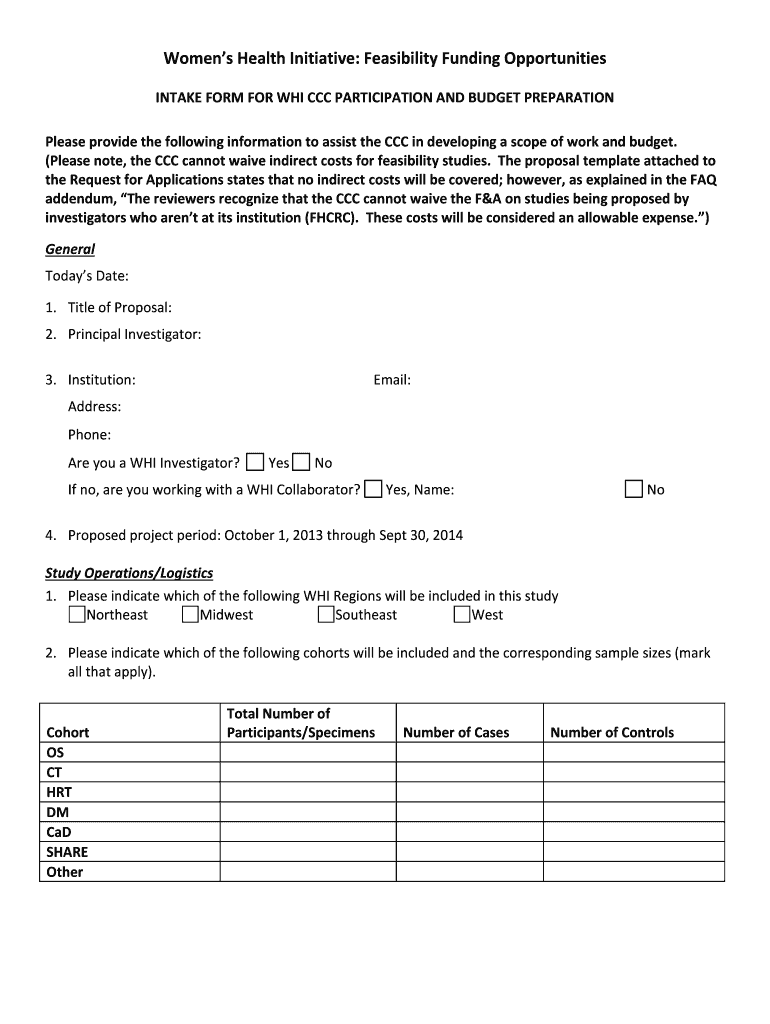
Get the free WHI Ancillary Study Intake form for Clinical Coordinating Center
Show details
Women's Health Initiative: Feasibility Funding Opportunities
INTAKE FORM FOR WHO CCC PARTICIPATION AND BUDGET PREPARATION
Please provide the following information to assist the CCC in developing a
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign whi ancillary study intake

Edit your whi ancillary study intake form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your whi ancillary study intake form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing whi ancillary study intake online
Follow the steps below to benefit from the PDF editor's expertise:
1
Create an account. Begin by choosing Start Free Trial and, if you are a new user, establish a profile.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit whi ancillary study intake. Text may be added and replaced, new objects can be included, pages can be rearranged, watermarks and page numbers can be added, and so on. When you're done editing, click Done and then go to the Documents tab to combine, divide, lock, or unlock the file.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
It's easier to work with documents with pdfFiller than you can have ever thought. Sign up for a free account to view.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out whi ancillary study intake

How to fill out whi ancillary study intake
01
To fill out the WHI Ancillary Study Intake, follow these steps:
02
Start by reviewing the WHI Ancillary Study Intake form and understanding the information it requests.
03
Gather all the necessary documents and information related to the intake, such as personal identification, medical history, and any relevant study-specific requirements.
04
Ensure that you have all the required supporting documents, if any, such as consent forms or previous study participation records.
05
Begin filling out the form by providing accurate and complete information in the specified fields. Ensure that you do not leave any mandatory fields blank.
06
Double-check your entries for any errors or inconsistencies. It's important to provide correct information to ensure the accuracy of the study.
07
If you have any questions or need assistance, reach out to the study coordinator or the designated contact person for the WHI Ancillary Study.
08
Once you have filled out the entire form, review it once again to ensure everything is accurate and complete.
09
Submit the WHI Ancillary Study Intake form as per the given instructions. This may involve submitting it online or mailing it to the designated address.
10
Keep a copy of the filled-out form for your records.
11
Wait for further instructions or contact from the WHI Ancillary Study team regarding your intake and potential participation.
Who needs whi ancillary study intake?
01
The WHI Ancillary Study intake is typically required for individuals who meet the study's eligibility criteria and are interested in participating in the specific ancillary study.
02
The exact eligibility requirements may vary depending on the objectives and protocols of each ancillary study within the WHI.
03
Generally, individuals who have been enrolled in the Women's Health Initiative (WHI) and meet the predetermined criteria for a particular ancillary study may need to fill out the WHI Ancillary Study Intake.
04
These individuals may include women of certain age groups, specific medical conditions, or those who fall within the targeted population of the ancillary study.
05
It is important to review the specific eligibility criteria and guidelines provided by the WHI Ancillary Study to determine if you need to fill out the intake form.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I make edits in whi ancillary study intake without leaving Chrome?
Add pdfFiller Google Chrome Extension to your web browser to start editing whi ancillary study intake and other documents directly from a Google search page. The service allows you to make changes in your documents when viewing them in Chrome. Create fillable documents and edit existing PDFs from any internet-connected device with pdfFiller.
How can I edit whi ancillary study intake on a smartphone?
The best way to make changes to documents on a mobile device is to use pdfFiller's apps for iOS and Android. You may get them from the Apple Store and Google Play. Learn more about the apps here. To start editing whi ancillary study intake, you need to install and log in to the app.
How do I fill out the whi ancillary study intake form on my smartphone?
Use the pdfFiller mobile app to fill out and sign whi ancillary study intake. Visit our website (https://edit-pdf-ios-android.pdffiller.com/) to learn more about our mobile applications, their features, and how to get started.
What is whi ancillary study intake?
WHI (Women's Health Initiative) ancillary study intake refers to the process of submitting information regarding secondary or additional studies that are conducted in conjunction with the main WHI research.
Who is required to file whi ancillary study intake?
Researchers, institutions, or organizations involved in conducting ancillary studies that are affiliated with the WHI are required to file the intake.
How to fill out whi ancillary study intake?
The WHI ancillary study intake form can usually be filled out online and requires specific details about the study, including its objectives, methodology, and timeline.
What is the purpose of whi ancillary study intake?
The purpose of WHI ancillary study intake is to ensure that all related research activities are documented and aligned with the main WHI study, as well as to maintain transparency and accountability.
What information must be reported on whi ancillary study intake?
Information such as the title of the study, principal investigator, funding sources, study design, and potential impact on participants must be reported on the WHI ancillary study intake form.
Fill out your whi ancillary study intake online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Whi Ancillary Study Intake is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.