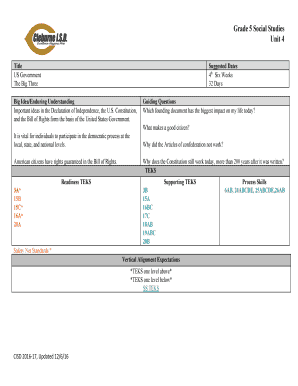
Get the free com CLIA# 05D0981414 Laboratory Director: Trieu Timothy D
Show details
... consent for genetic testing to be performed and the signed consent form is on file. ... For single site analyses, insurance reverification will not be performed unless .... Billing Questions:
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign com clia 05d0981414 laboratory

Edit your com clia 05d0981414 laboratory form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your com clia 05d0981414 laboratory form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing com clia 05d0981414 laboratory online
Use the instructions below to start using our professional PDF editor:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit com clia 05d0981414 laboratory. Add and replace text, insert new objects, rearrange pages, add watermarks and page numbers, and more. Click Done when you are finished editing and go to the Documents tab to merge, split, lock or unlock the file.
4
Save your file. Select it from your list of records. Then, move your cursor to the right toolbar and choose one of the exporting options. You can save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud, among other things.
It's easier to work with documents with pdfFiller than you could have believed. You may try it out for yourself by signing up for an account.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out com clia 05d0981414 laboratory

How to fill out com clia 05d0981414 laboratory:
01
Start by gathering all required information and documents, such as patient details, test requisition forms, and laboratory test protocols.
02
Carefully read and understand the instructions provided on the com clia 05d0981414 laboratory form. Familiarize yourself with the specific requirements and guidelines.
03
Begin filling out the laboratory form by entering the patient's personal information accurately. This typically includes the patient's full name, date of birth, address, and contact details.
04
Proceed to fill in the relevant medical history of the patient, if required. This may involve providing information about any existing medical conditions, previous surgeries, or current medications being taken.
05
Make sure to provide clear and concise details regarding the specific tests to be conducted in the laboratory. Indicate the test names, codes, or any other identifiers mentioned on the com clia 05d0981414 laboratory form.
06
Follow any specific instructions provided on the form for sample collection, storage, or transportation. Ensure that you adhere to the recommended procedures to maintain the integrity of the samples.
07
Double-check all the information filled on the com clia 05d0981414 laboratory form for accuracy and completeness. Any errors or omissions could affect the test results or delay the processing.
08
Once you have thoroughly reviewed the form, sign and date it as per the required fields. This signature serves as an acknowledgment of the accuracy and completeness of the information provided.
09
Submit the completed com clia 05d0981414 laboratory form along with the required samples and any supporting documents to the designated laboratory as instructed.
Who needs com clia 05d0981414 laboratory?
01
Patients who require specific diagnostic tests to assess their medical conditions or monitor their health status may need the services of com clia 05d0981414 laboratory.
02
Physicians, healthcare providers, or medical professionals who order diagnostic tests to aid in the diagnosis, treatment, or management of their patients may need the services of com clia 05d0981414 laboratory.
03
Medical research institutions or pharmaceutical companies conducting clinical trials or studies that involve laboratory testing may require the services of com clia 05d0981414 laboratory.
04
Government agencies or regulatory bodies responsible for monitoring public health or enforcing health regulations may need the services of com clia 05d0981414 laboratory to assess compliance or investigate potential health-related issues.
05
Insurance companies or third-party payers may request certain laboratory tests from com clia 05d0981414 laboratory to evaluate claims or assess eligibility for coverage.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is com clia 05d0981414 laboratory?
COM CLIA 05D0981414 laboratory is a Clinical Laboratory Improvement Amendments (CLIA) number assigned to a specific laboratory for regulatory purposes.
Who is required to file com clia 05d0981414 laboratory?
The laboratory facility or organization that holds the CLIA number 05D0981414 is required to file com CLIA 05D0981414 laboratory.
How to fill out com clia 05d0981414 laboratory?
To fill out com CLIA 05D0981414 laboratory, the laboratory must report all necessary information as required by CLIA regulations.
What is the purpose of com clia 05d0981414 laboratory?
The purpose of com CLIA 05D0981414 laboratory is to ensure that the laboratory meets quality standards and complies with CLIA regulations to provide accurate and reliable testing.
What information must be reported on com clia 05d0981414 laboratory?
The information reported on com CLIA 05D0981414 laboratory includes testing procedures, quality control measures, personnel qualifications, and proficiency testing results.
Can I create an eSignature for the com clia 05d0981414 laboratory in Gmail?
You can easily create your eSignature with pdfFiller and then eSign your com clia 05d0981414 laboratory directly from your inbox with the help of pdfFiller’s add-on for Gmail. Please note that you must register for an account in order to save your signatures and signed documents.
How do I edit com clia 05d0981414 laboratory on an iOS device?
Use the pdfFiller mobile app to create, edit, and share com clia 05d0981414 laboratory from your iOS device. Install it from the Apple Store in seconds. You can benefit from a free trial and choose a subscription that suits your needs.
Can I edit com clia 05d0981414 laboratory on an Android device?
You can make any changes to PDF files, such as com clia 05d0981414 laboratory, with the help of the pdfFiller mobile app for Android. Edit, sign, and send documents right from your mobile device. Install the app and streamline your document management wherever you are.
Fill out your com clia 05d0981414 laboratory online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Com Clia 05D0981414 Laboratory is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.