FDA 3514 2017 free printable template
Show details
An agency may not conduct or sponsor, and a person is not required to respond to, a collection of information unless it displays a currently valid OMB control number.
pdfFiller is not affiliated with any government organization
Get, Create, Make and Sign FDA 3514

Edit your FDA 3514 form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your FDA 3514 form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit FDA 3514 online
Use the instructions below to start using our professional PDF editor:
1
Log in. Click Start Free Trial and create a profile if necessary.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit FDA 3514. Replace text, adding objects, rearranging pages, and more. Then select the Documents tab to combine, divide, lock or unlock the file.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
FDA 3514 Form Versions
Version
Form Popularity
Fillable & printabley
How to fill out FDA 3514

How to fill out FDA 3514
01
Obtain the FDA Form 3514 from the official FDA website or necessary agency.
02
Read the instructions provided on the form carefully to understand the requirements.
03
Fill in the applicant information, including name, address, and contact details.
04
Provide the product information, including the specific drug or biological product name.
05
Indicate the proposal type (e.g., new application, amendment, etc.) in the appropriate field.
06
Include any relevant manufacturing information if applicable.
07
Review the completed form for accuracy and completeness.
08
Submit the form to the appropriate FDA office according to submission guidelines.
Who needs FDA 3514?
01
Pharmaceutical companies seeking FDA approval for new drugs or biological products.
02
Researchers submitting IND applications for investigational new drugs.
03
Manufacturers of biological products who need to comply with FDA regulations.
04
Organizations involved in clinical trials requiring FDA oversight.
Fill
form
: Try Risk Free






People Also Ask about
What is FDA form 3514?
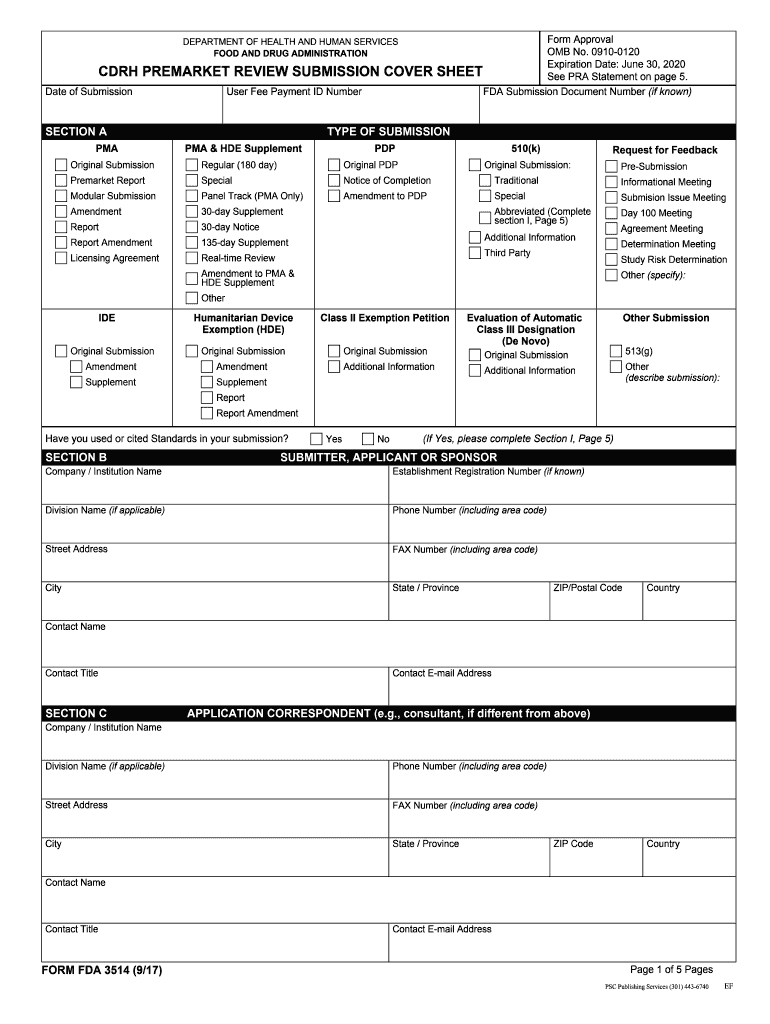
Form 3514) The CDRH Premarket Review Submission Cover Sheet11 is a voluntary form used to help provide basic administrative information for all types of premarket notification submissions.
What are the requirements for 510k reporting?
A 510(k) requires demonstration of substantial equivalence to another legally U.S. marketed device. Substantial equivalence means that the new device is as safe and effective as the predicate. the information submitted to FDA demonstrates that the device is as safe and effective as the legally marketed device.
Is FDA form 3514 required?
FDA Form 3514. The use of this form is optional. If you choose not to use the form, ensure that the relevant information is contained in the cover letter:.
What is FDA Form 3514?
Form 3514) The CDRH Premarket Review Submission Cover Sheet11 is a voluntary form used to help provide basic administrative information for all types of premarket notification submissions.
What is form 3454?
Form FDA 3454, or the Financial Certification or Disclosure Statement, is used to submit information regarding clinical investigators who participated in the clinical studies. If no clinical studies were performed, simply state: “no clinical studies were performed to test this device.”
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
Can I sign the FDA 3514 electronically in Chrome?
Yes. You can use pdfFiller to sign documents and use all of the features of the PDF editor in one place if you add this solution to Chrome. In order to use the extension, you can draw or write an electronic signature. You can also upload a picture of your handwritten signature. There is no need to worry about how long it takes to sign your FDA 3514.
Can I edit FDA 3514 on an iOS device?
Create, edit, and share FDA 3514 from your iOS smartphone with the pdfFiller mobile app. Installing it from the Apple Store takes only a few seconds. You may take advantage of a free trial and select a subscription that meets your needs.
How do I fill out FDA 3514 on an Android device?
On Android, use the pdfFiller mobile app to finish your FDA 3514. Adding, editing, deleting text, signing, annotating, and more are all available with the app. All you need is a smartphone and internet.
What is FDA 3514?
FDA 3514 is a form used by certain establishments to report the manufacturing, processing, packaging, or holding of food products to the Food and Drug Administration (FDA).
Who is required to file FDA 3514?
Establishments that engage in the manufacturing, processing, packaging, or holding of food products and are subject to FDA regulations are required to file FDA 3514.
How to fill out FDA 3514?
To fill out FDA 3514, the responsible party must provide specific information about the establishment, including name, address, and details about the operations conducted, ensuring all fields are accurately completed.
What is the purpose of FDA 3514?
The purpose of FDA 3514 is to collect essential information on food facilities to ensure compliance with FDA regulations and facilitate the agency's ability to oversee food safety.
What information must be reported on FDA 3514?
Information that must be reported on FDA 3514 includes the establishment's name, address, type of operations, and details regarding the products manufactured or handled.
Fill out your FDA 3514 online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

FDA 3514 is not the form you're looking for?Search for another form here.
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.
























