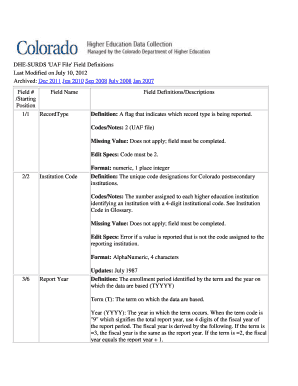
Get the free CTD, CEP and Active Substance Master File - European ...
Show details
ECA Certified Regulatory Affairs Manager Course* Choose 2 out of 4 Parallel Workshops ?? Stability Studies and Establishing the Retest Date ?? Description of the Manufacturing Process ?? How to Compile
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign ctd cep and active

Edit your ctd cep and active form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your ctd cep and active form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing ctd cep and active online
In order to make advantage of the professional PDF editor, follow these steps below:
1
Log in to account. Click on Start Free Trial and sign up a profile if you don't have one.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit ctd cep and active. Add and change text, add new objects, move pages, add watermarks and page numbers, and more. Then click Done when you're done editing and go to the Documents tab to merge or split the file. If you want to lock or unlock the file, click the lock or unlock button.
4
Get your file. Select the name of your file in the docs list and choose your preferred exporting method. You can download it as a PDF, save it in another format, send it by email, or transfer it to the cloud.
With pdfFiller, it's always easy to work with documents. Check it out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out ctd cep and active

How to fill out CTD CEP and Active:
01
Gather all the necessary information: Before filling out the CTD CEP and Active form, ensure that you have all the required information. This may include details about the product, its composition, manufacturing process, stability data, clinical studies, and safety information.
02
Start with the cover letter: Begin by writing a cover letter that introduces the submission and provides any relevant background information. Clearly state the purpose of the submission and include any special considerations or requests.
03
Complete the administrative information: Fill in the administrative sections of the form, such as the applicant's details, reference number, and submission date. Make sure all the provided information is accurate and up-to-date.
04
Provide detailed product information: In the sections dedicated to product information, provide a comprehensive description of the product, including its intended use, dosage form, route of administration, and any other relevant details. Include information about any excipients or additives used in the formulation.
05
Submit quality data and documentation: Attach all the required quality data and documentation, such as manufacturing process details, specifications, stability studies, and analytical test methods. This information should support the safety, quality, and efficacy of the product.
06
Include non-clinical and clinical data: If applicable, provide non-clinical and clinical data to support the product's safety and effectiveness. Include information about preclinical studies, pharmacokinetics, pharmacodynamics, and results from any clinical trials conducted.
07
Cross-reference with relevant guidelines: Ensure that your submission is in line with the appropriate regulatory guidelines. Cross-reference the sections of the CTD CEP and Active form with the relevant guidelines to ensure complete compliance.
08
Review and proofread: Before finalizing your submission, thoroughly review the completed form, attachments, and any supporting documentation. Check for any errors or inconsistencies and make necessary corrections.
Who needs CTD CEP and Active?
CTD CEP and Active are necessary for pharmaceutical companies and organizations involved in the research, development, and registration of medicinal products. This could include:
01
Pharmaceutical manufacturers: Companies that produce and market pharmaceutical products are required to fill out CTD CEP and Active as part of the registration process. These documents provide the necessary information for regulatory authorities to assess the safety, quality, and efficacy of the products.
02
Regulatory authorities: Government agencies responsible for regulating and authorizing the sale of medicinal products require CTD CEP and Active submissions to evaluate and approve new drugs or variations to existing products. These authorities review the information provided to ensure compliance with regulatory standards and protect public health.
03
Contract research organizations (CROs): CROs may assist pharmaceutical companies in preparing and submitting CTD CEP and Active documentation. These organizations have expertise in regulatory affairs and can guide companies through the complex process of filling out the forms and compiling the necessary data.
04
Pharmacists and healthcare professionals: Pharmacists and healthcare professionals may refer to CTD CEP and Active information when assessing the quality and safety of pharmaceutical products. This information helps them understand the composition, indications, contraindications, and potential adverse effects of the medications they dispense or prescribe.
Remember, the specific requirements for CTD CEP and Active submissions may vary depending on the country or region. It is crucial to consult the applicable regulations and guidelines to ensure compliance and a successful submission.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is ctd cep and active?
CTD CEP stands for Common Technical Document Certificate of Suitability for Product Manufacture. ACTIVE stands for Active Substance Master File. These are documents required for pharmaceutical substances.
Who is required to file ctd cep and active?
Pharmaceutical companies and manufacturers are required to file CTD CEP and ACTIVE for their products.
How to fill out ctd cep and active?
CTD CEP and ACTIVE forms can be filled out online through regulatory authorities or submitted physically with required documentation.
What is the purpose of ctd cep and active?
The purpose of CTD CEP and ACTIVE is to ensure the quality, safety, and efficacy of pharmaceutical substances.
What information must be reported on ctd cep and active?
Information regarding the manufacturing process, quality control, safety data, and efficacy studies must be reported on CTD CEP and ACTIVE.
How do I make edits in ctd cep and active without leaving Chrome?
Adding the pdfFiller Google Chrome Extension to your web browser will allow you to start editing ctd cep and active and other documents right away when you search for them on a Google page. People who use Chrome can use the service to make changes to their files while they are on the Chrome browser. pdfFiller lets you make fillable documents and make changes to existing PDFs from any internet-connected device.
Can I create an electronic signature for signing my ctd cep and active in Gmail?
It's easy to make your eSignature with pdfFiller, and then you can sign your ctd cep and active right from your Gmail inbox with the help of pdfFiller's add-on for Gmail. This is a very important point: You must sign up for an account so that you can save your signatures and signed documents.
How do I edit ctd cep and active on an iOS device?
You can. Using the pdfFiller iOS app, you can edit, distribute, and sign ctd cep and active. Install it in seconds at the Apple Store. The app is free, but you must register to buy a subscription or start a free trial.
Fill out your ctd cep and active online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Ctd Cep And Active is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.