
Get the free Biological Product and HCT/P Deviation Reports - fda.gov
Show details
Department of Health and Human Services
Food and Drug Administration
Center for Drug Evaluation and Research
Office of Surveillance and Epidemiology
Office of Medication Error Prevention and Risk
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign biological product and hctp
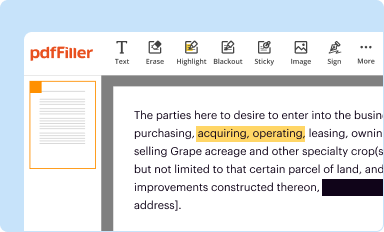
Edit your biological product and hctp form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.
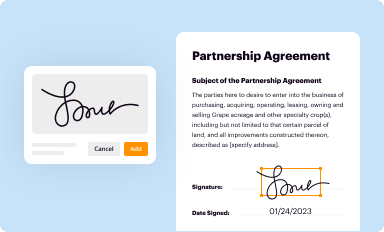
Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your biological product and hctp form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit biological product and hctp online
To use the professional PDF editor, follow these steps below:
1
Log into your account. If you don't have a profile yet, click Start Free Trial and sign up for one.
2
Upload a document. Select Add New on your Dashboard and transfer a file into the system in one of the following ways: by uploading it from your device or importing from the cloud, web, or internal mail. Then, click Start editing.
3
Edit biological product and hctp. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
pdfFiller makes dealing with documents a breeze. Create an account to find out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out biological product and hctp
How to fill out biological product and hctp
01
To fill out a biological product and HCTP, follow these steps:
02
Gather all necessary information and documentation related to the biological product and HCTP, such as product labeling, storage requirements, and administration guidelines.
03
Ensure proper cleanliness and hygiene by sanitizing your hands and any equipment or surfaces that will come into contact with the biological product and HCTP.
04
Verify that the biological product and HCTP are in proper condition and have not expired. Check for any visible signs of damage or deterioration.
05
Carefully read and understand the instructions provided with the biological product and HCTP. Follow them precisely to ensure appropriate handling and administration.
06
Prepare the necessary equipment and supplies, such as syringes, needles, and diluents, according to the instructions provided.
07
If required, reconstitute or dilute the biological product as directed, taking into account the appropriate sterile technique and recommended storage conditions.
08
Use aseptic technique when handling the biological product and HCTP, ensuring that all instruments and surfaces remain free from contamination.
09
Administer the biological product and HCTP according to the route and dose recommended. Follow any specific guidelines or protocols provided by healthcare professionals.
10
Dispose of any unused or expired biological product and HCTP properly, following the guidelines for medical waste management and disposal.
11
Record and document all relevant information, including the date, time, dosage administered, and any observations or adverse reactions.
12
Store any remaining biological product and HCTP as per the instructions, ensuring proper temperature, light exposure, and security.
13
Seek professional guidance or consultation if you have any doubts or concerns regarding the filling out of the biological product and HCTP.
Who needs biological product and hctp?
01
Biological products and HCTPs are needed by various individuals and groups, including:
02
- Patients with specific medical conditions or diseases that can be treated or managed using biological products or HCTPs.
03
- Healthcare professionals, such as doctors, nurses, and pharmacists, who administer or prescribe biological products and HCTPs to patients.
04
- Researchers and scientists involved in studying and developing new biological products and HCTPs for medical purposes.
05
- Regulatory authorities and government agencies responsible for approving, monitoring, and ensuring the quality and safety of biological products and HCTPs.
06
- Pharmaceutical companies and manufacturers who produce and distribute biological products and HCTPs.
07
- Donors and recipients involved in the process of tissue or cell transplantation, which often requires the use of biological products and HCTPs.
08
- Veterinary practitioners who treat animals or perform veterinary procedures involving biological products and HCTPs.
09
- Individuals participating in clinical trials or research studies that investigate the efficacy and safety of new biological products and HCTPs.
10
- Public health organizations and institutions engaged in the prevention, control, and management of diseases that may require the use of biological products and HCTPs.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I edit biological product and hctp in Chrome?
Install the pdfFiller Chrome Extension to modify, fill out, and eSign your biological product and hctp, which you can access right from a Google search page. Fillable documents without leaving Chrome on any internet-connected device.
How can I edit biological product and hctp on a smartphone?
The best way to make changes to documents on a mobile device is to use pdfFiller's apps for iOS and Android. You may get them from the Apple Store and Google Play. Learn more about the apps here. To start editing biological product and hctp, you need to install and log in to the app.
Can I edit biological product and hctp on an iOS device?
Yes, you can. With the pdfFiller mobile app, you can instantly edit, share, and sign biological product and hctp on your iOS device. Get it at the Apple Store and install it in seconds. The application is free, but you will have to create an account to purchase a subscription or activate a free trial.
What is biological product and hctp?
Biological products are medical products made from living organisms or their components, such as vaccines, blood, and tissues. HCT/P stands for human cells, tissues, and cellular and tissue-based products, which are regulated by the FDA.
Who is required to file biological product and hctp?
Manufacturers, distributors, and importers of biological products and HCT/Ps are required to file with the FDA.
How to fill out biological product and hctp?
The filing of biological products and HCT/Ps typically requires submitting an application or report to the FDA, including detailed information about the product and its manufacturing process.
What is the purpose of biological product and hctp?
The purpose of regulating biological products and HCT/Ps is to ensure their safety, efficacy, and quality for the protection of public health.
What information must be reported on biological product and hctp?
Information such as product description, manufacturing process, quality control measures, adverse events, and labeling must be reported for biological products and HCT/Ps.
Fill out your biological product and hctp online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Biological Product And Hctp is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















