Get the free IRB Forms and ProtocolsResearch - New Jersey Institute of ...
Show details
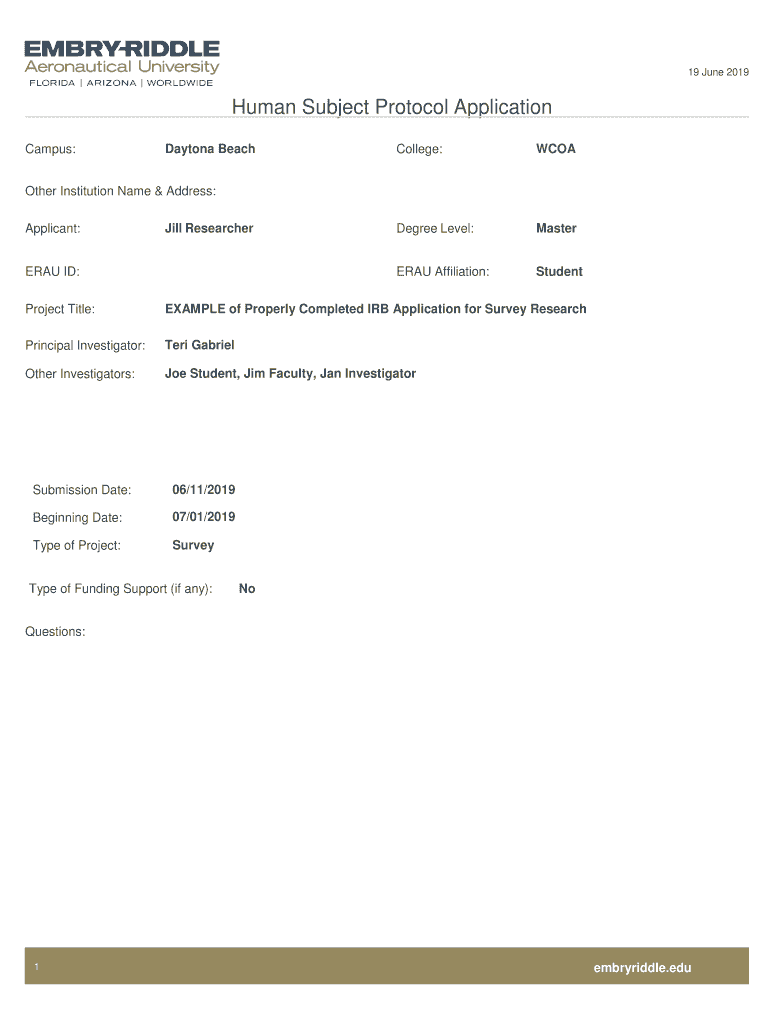
19 June 2019Human Subject Protocol Application Campus:Daytona BeachCollege:Nondegree Level:Mastered Affiliation:StudentOther Institution Name & Address: Applicant:Jill ResearcherERAU ID: Project Title:EXAMPLE
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign irb forms and protocolsresearch

Edit your irb forms and protocolsresearch form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your irb forms and protocolsresearch form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit irb forms and protocolsresearch online
Follow the steps down below to benefit from the PDF editor's expertise:
1
Sign into your account. If you don't have a profile yet, click Start Free Trial and sign up for one.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit irb forms and protocolsresearch. Text may be added and replaced, new objects can be included, pages can be rearranged, watermarks and page numbers can be added, and so on. When you're done editing, click Done and then go to the Documents tab to combine, divide, lock, or unlock the file.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
pdfFiller makes dealing with documents a breeze. Create an account to find out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out irb forms and protocolsresearch

How to fill out irb forms and protocolsresearch
01
To fill out IRB forms and protocols for research, follow these steps:
02
Determine the type of research you will be conducting and the form you need to fill out. IRB forms vary depending on the nature of the study.
03
Read the instructions and guidelines provided with the form. Familiarize yourself with the requirements and expectations of the IRB.
04
Gather all necessary information and supporting documents before you begin filling out the form. This may include your research proposal, participant consent forms, recruitment materials, etc.
05
Start by providing basic information about yourself, the research team, and the institution where the study will be conducted. Fill in all the required fields accurately.
06
Provide a clear and concise statement of the research objectives and the methodology that will be used. Clearly explain how you intend to protect the rights and welfare of participants.
07
Specify the criteria for participant selection, recruitment methods, and the informed consent process. Describe any potential risks and benefits associated with the study.
08
Include details about data collection and analysis procedures, as well as plans for maintaining confidentiality and data security.
09
If your research involves vulnerable populations or sensitive topics, outline additional safeguards you will implement.
10
Review the completed form to ensure all necessary information has been provided and that it is free of errors.
11
Submit the form and any required supporting documents to the appropriate IRB office for review and approval. Follow up with any additional requests or revisions as necessary.
Who needs irb forms and protocolsresearch?
01
Anyone involved in research that involves human participants needs to fill out IRB forms and protocols. This includes researchers from various fields such as medicine, psychology, social sciences, and more. IRB forms and protocols ensure that ethical standards are upheld, and the rights and welfare of research participants are protected. These forms are typically required by academic institutions, funding agencies, and regulatory bodies to ensure compliance with ethical guidelines and legal requirements.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
Can I edit irb forms and protocolsresearch on an iOS device?
You can. Using the pdfFiller iOS app, you can edit, distribute, and sign irb forms and protocolsresearch. Install it in seconds at the Apple Store. The app is free, but you must register to buy a subscription or start a free trial.
How do I complete irb forms and protocolsresearch on an iOS device?
Download and install the pdfFiller iOS app. Then, launch the app and log in or create an account to have access to all of the editing tools of the solution. Upload your irb forms and protocolsresearch from your device or cloud storage to open it, or input the document URL. After filling out all of the essential areas in the document and eSigning it (if necessary), you may save it or share it with others.
How do I edit irb forms and protocolsresearch on an Android device?
You can edit, sign, and distribute irb forms and protocolsresearch on your mobile device from anywhere using the pdfFiller mobile app for Android; all you need is an internet connection. Download the app and begin streamlining your document workflow from anywhere.
What is irb forms and protocolsresearch?
IRB forms and protocolsresearch are documents used to outline the plan for conducting research involving human subjects.
Who is required to file irb forms and protocolsresearch?
Researchers and institutions conducting research involving human subjects are required to file IRB forms and protocols.
How to fill out irb forms and protocolsresearch?
IRB forms and protocolsresearch should be filled out by providing detailed information about the research plan, potential risks to participants, and how participant confidentiality will be protected.
What is the purpose of irb forms and protocolsresearch?
The purpose of IRB forms and protocolsresearch is to ensure that research involving human subjects is conducted ethically and in compliance with regulations to protect participants.
What information must be reported on irb forms and protocolsresearch?
IRB forms and protocolsresearch must include details about the research methodology, potential risks to participants, informed consent procedures, and plans for data management.
Fill out your irb forms and protocolsresearch online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Irb Forms And Protocolsresearch is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















