Get the free A study of transobturator tape in stress urinary incontinence - bsug org
Show details
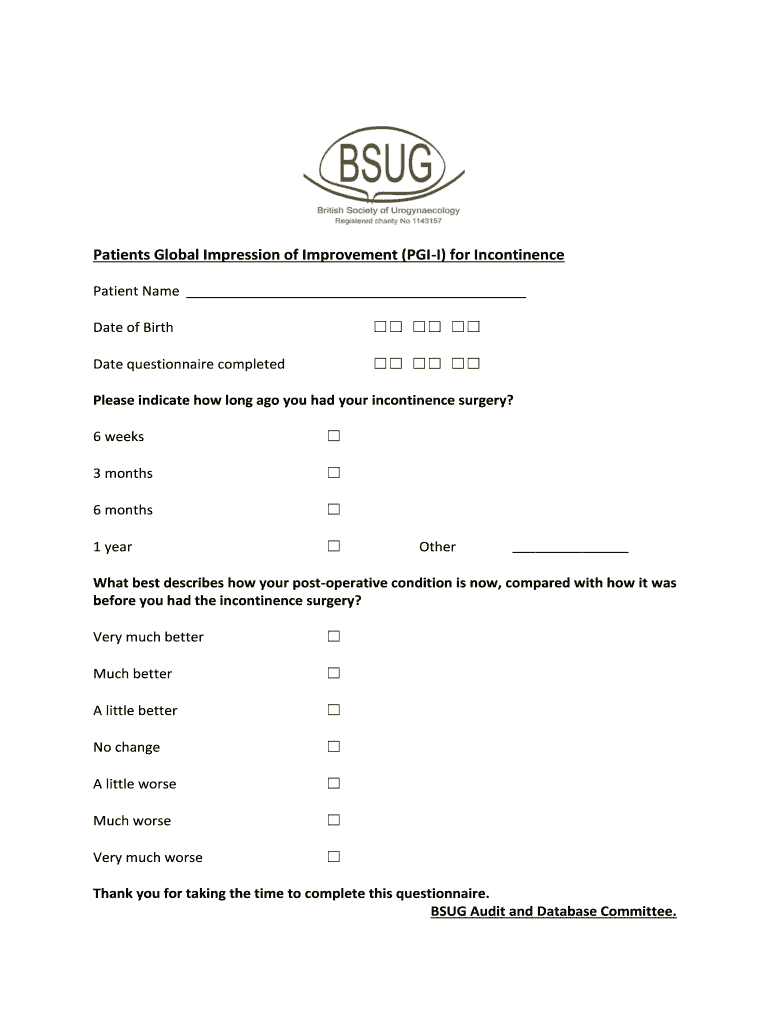
Patients Global Impression of Improvement (PGI) for Incontinence Patient Name Date of Birth Date questionnaire completed Please indicate how long ago you had your incontinence surgery? 6 weeks3 months6
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign a study of transobturator

Edit your a study of transobturator form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your a study of transobturator form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit a study of transobturator online
Follow the guidelines below to benefit from the PDF editor's expertise:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Upload a file. Select Add New on your Dashboard and upload a file from your device or import it from the cloud, online, or internal mail. Then click Edit.
3
Edit a study of transobturator. Replace text, adding objects, rearranging pages, and more. Then select the Documents tab to combine, divide, lock or unlock the file.
4
Get your file. When you find your file in the docs list, click on its name and choose how you want to save it. To get the PDF, you can save it, send an email with it, or move it to the cloud.
It's easier to work with documents with pdfFiller than you can have believed. Sign up for a free account to view.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out a study of transobturator

How to fill out a study of transobturator
01
To fill out a study of transobturator, follow these steps:
02
Gather the necessary equipment, including a transobturator sling kit, gloves, drape, antiseptic solution, and local anesthesia.
03
Ensure the patient is in a lithotomy position with her legs in stirrups.
04
Cleanse and drape the area around the patient's urethra, vagina, and inner thighs using the antiseptic solution.
05
Administer local anesthesia to minimize discomfort or pain during the procedure.
06
Make a small incision on each side of the patient's groin, just below the inguinal crease, to gain access to the obturator foramen.
07
Insert the transobturator sling through the incisions and guide it along the lateral walls of the pelvis, avoiding any major blood vessels or nerves.
08
Adjust the tension of the sling to provide support to the urethra without causing obstruction or discomfort.
09
Close the incisions using sutures or adhesive skin closure strips.
10
Ensure proper placement and function of the transobturator sling through cystoscopy or other imaging techniques.
11
Provide post-procedure instructions and follow-up care to the patient.
Who needs a study of transobturator?
01
A study of transobturator is typically needed for individuals who are experiencing symptoms of stress urinary incontinence (SUI).
02
This condition is characterized by the involuntary leakage of urine during physical activities, such as coughing, sneezing, laughing, or exercising.
03
Women who have weakened pelvic floor muscles or a weak urethral sphincter may require a study of transobturator to evaluate their condition and determine the most appropriate treatment.
04
It is recommended to consult with a healthcare professional, such as a urologist or urogynecologist, to determine if a study of transobturator is necessary for an individual.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I edit a study of transobturator from Google Drive?
It is possible to significantly enhance your document management and form preparation by combining pdfFiller with Google Docs. This will allow you to generate papers, amend them, and sign them straight from your Google Drive. Use the add-on to convert your a study of transobturator into a dynamic fillable form that can be managed and signed using any internet-connected device.
Can I sign the a study of transobturator electronically in Chrome?
As a PDF editor and form builder, pdfFiller has a lot of features. It also has a powerful e-signature tool that you can add to your Chrome browser. With our extension, you can type, draw, or take a picture of your signature with your webcam to make your legally-binding eSignature. Choose how you want to sign your a study of transobturator and you'll be done in minutes.
Can I create an eSignature for the a study of transobturator in Gmail?
When you use pdfFiller's add-on for Gmail, you can add or type a signature. You can also draw a signature. pdfFiller lets you eSign your a study of transobturator and other documents right from your email. In order to keep signed documents and your own signatures, you need to sign up for an account.
What is a study of transobturator?
A study of transobturator is a type of medical research study that focuses on the use of transobturator slings for treating urinary incontinence.
Who is required to file a study of transobturator?
Medical practitioners and researchers who are conducting studies on the use of transobturator slings are required to file a study of transobturator.
How to fill out a study of transobturator?
To fill out a study of transobturator, researchers must provide detailed information about the study design, participant demographics, outcomes, and any adverse events related to the use of transobturator slings.
What is the purpose of a study of transobturator?
The purpose of a study of transobturator is to evaluate the safety and effectiveness of transobturator slings in treating urinary incontinence.
What information must be reported on a study of transobturator?
Researchers must report information about the study design, participant demographics, outcomes, adverse events, and any other relevant data related to the use of transobturator slings.
Fill out your a study of transobturator online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

A Study Of Transobturator is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















