Get the free EDRN Specimen Reference Sets: Paving the Way for Rapid ...
Show details
V
E
e
d
r
n
away
a
y
a
d
y
r
e
eV
u
t
o
...
B
a
g
n
i
k
l
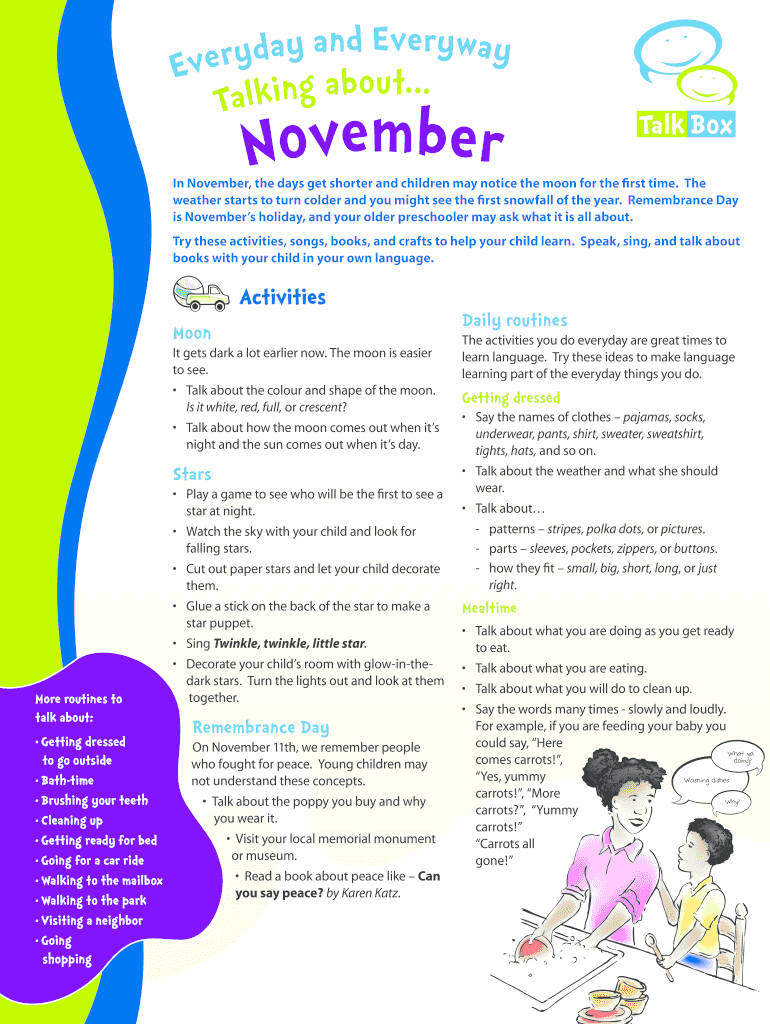
TaNovemberTalk Boxing November, the days get shorter and children may notice the moon for the first time. The
weather starts to turn colder,
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign edrn specimen reference sets

Edit your edrn specimen reference sets form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your edrn specimen reference sets form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit edrn specimen reference sets online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit edrn specimen reference sets. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Get your file. When you find your file in the docs list, click on its name and choose how you want to save it. To get the PDF, you can save it, send an email with it, or move it to the cloud.
It's easier to work with documents with pdfFiller than you could have ever thought. Sign up for a free account to view.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out edrn specimen reference sets

How to fill out edrn specimen reference sets
01
Begin by gathering all necessary information for the specimen reference set, such as specimen types, identifiers, and associated metadata.
02
Create a detailed template or form to capture the required information for each specimen in the reference set. This may include fields for specimen ID, collection date, sample processing details, etc.
03
Use the template to fill out the information for each specimen in the reference set. Be sure to provide accurate and complete data.
04
Consider any specific requirements or guidelines for the reference set, such as inclusion/exclusion criteria or standardized terminology.
05
Validate the filled-out information for each specimen to ensure accuracy and consistency.
06
Once all specimens have been filled out and validated, compile the information into a cohesive reference set.
07
Make sure to properly document the reference set, including any relevant metadata or annotations.
08
Share the reference set with the intended users or stakeholders, such as researchers, clinicians, or quality assurance personnel.
09
Continuously update and maintain the reference set as new specimens become available or as changes in the data occur.
10
Periodically review the reference set to ensure its relevance and accuracy.
Who needs edrn specimen reference sets?
01
Researchers conducting studies or experiments that involve specimen analysis may need edrn specimen reference sets for quality control, comparison, or standardization purposes.
02
Clinicians or pathologists who need to compare patient specimens to a known reference set for diagnostic or prognostic purposes may also require edrn specimen reference sets.
03
Laboratories or research institutions that aim to establish or validate new testing methods or procedures may use edrn specimen reference sets to benchmark their results against a known standard.
04
Quality assurance personnel responsible for monitoring and improving laboratory processes and procedures may benefit from edrn specimen reference sets for performance evaluation.
05
Regulatory agencies or governing bodies may require edrn specimen reference sets as part of the validation or certification process for certain diagnostic tests or assays.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I manage my edrn specimen reference sets directly from Gmail?
The pdfFiller Gmail add-on lets you create, modify, fill out, and sign edrn specimen reference sets and other documents directly in your email. Click here to get pdfFiller for Gmail. Eliminate tedious procedures and handle papers and eSignatures easily.
How can I edit edrn specimen reference sets on a smartphone?
Using pdfFiller's mobile-native applications for iOS and Android is the simplest method to edit documents on a mobile device. You may get them from the Apple App Store and Google Play, respectively. More information on the apps may be found here. Install the program and log in to begin editing edrn specimen reference sets.
How do I edit edrn specimen reference sets on an Android device?
The pdfFiller app for Android allows you to edit PDF files like edrn specimen reference sets. Mobile document editing, signing, and sending. Install the app to ease document management anywhere.
What is edrn specimen reference sets?
The EDRN Specimen Reference Sets are a collection of biological specimens used for research purposes.
Who is required to file edrn specimen reference sets?
Researchers and institutions conducting research that involve specimen analysis are required to file EDRN Specimen Reference Sets.
How to fill out edrn specimen reference sets?
EDRN Specimen Reference Sets can be filled out by providing detailed information about the specimens being used in the research.
What is the purpose of edrn specimen reference sets?
The purpose of EDRN Specimen Reference Sets is to provide standardized reference materials for researchers to use in their studies.
What information must be reported on edrn specimen reference sets?
Information such as specimen type, source, processing methods, and storage conditions must be reported on EDRN Specimen Reference Sets.
Fill out your edrn specimen reference sets online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Edrn Specimen Reference Sets is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















