Get the free clinical study document tracking log template - Smartsheet
Show details
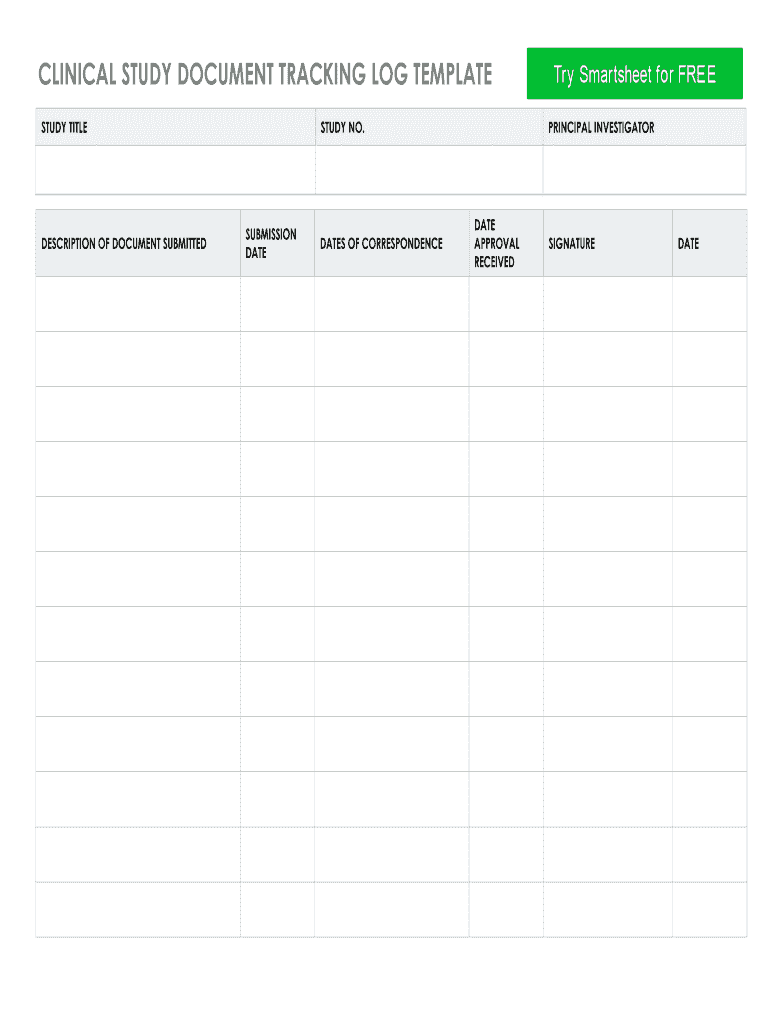
CLINICAL STUDY DOCUMENT TRACKING LOG TEMPLATE
STUDY TITLEDESCRIPTION OF DOCUMENT SUBMITTEDSTUDY NO.SUBMISSION
ANTEDATES OF CORRESPONDENCEPRINCIPAL INVESTIGATORDATE
APPROVAL
RECEIVEDSIGNATUREDATEDISCLAIMER
Any
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign clinical study document tracking

Edit your clinical study document tracking form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your clinical study document tracking form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit clinical study document tracking online
To use the professional PDF editor, follow these steps below:
1
Log in to your account. Start Free Trial and register a profile if you don't have one.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit clinical study document tracking. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
With pdfFiller, it's always easy to work with documents. Try it out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out clinical study document tracking

How to fill out clinical study document tracking
01
Begin by gathering all necessary documents for the clinical study, such as informed consent forms, case report forms, and study protocols.
02
Create a systematic tracking system, either electronically or physically, to keep records of all the documents involved in the study.
03
Assign unique identifiers or codes to each document to ensure easy tracking and retrieval.
04
Develop a comprehensive filing system or database to organize the documents based on categories, such as study phase, document type, or date.
05
Regularly update the tracking system with any new or revised documents to maintain accuracy and completeness.
06
Implement a secure backup system to protect the documents from loss or damage.
07
Train the appropriate personnel or team members on how to use the tracking system effectively.
08
Conduct periodic audits or reviews of the tracking system to verify its effectiveness and make necessary improvements.
09
Continuously monitor and track the progress of the clinical study by retrieving and updating the relevant documents in the tracking system.
10
Adhere to regulatory requirements and guidelines when handling and tracking clinical study documents.
Who needs clinical study document tracking?
01
Clinical research organizations
02
Pharmaceutical companies
03
Biotechnology companies
04
Academic research institutions
05
Government agencies
06
Contract research organizations
07
Investigators and research sites
08
Ethics committees
09
Medical professionals involved in clinical trials
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I send clinical study document tracking for eSignature?
clinical study document tracking is ready when you're ready to send it out. With pdfFiller, you can send it out securely and get signatures in just a few clicks. PDFs can be sent to you by email, text message, fax, USPS mail, or notarized on your account. You can do this right from your account. Become a member right now and try it out for yourself!
How can I edit clinical study document tracking on a smartphone?
The best way to make changes to documents on a mobile device is to use pdfFiller's apps for iOS and Android. You may get them from the Apple Store and Google Play. Learn more about the apps here. To start editing clinical study document tracking, you need to install and log in to the app.
How do I complete clinical study document tracking on an Android device?
Use the pdfFiller app for Android to finish your clinical study document tracking. The application lets you do all the things you need to do with documents, like add, edit, and remove text, sign, annotate, and more. There is nothing else you need except your smartphone and an internet connection to do this.
What is clinical study document tracking?
Clinical study document tracking is the process of monitoring and managing the various documents and records related to a clinical study.
Who is required to file clinical study document tracking?
The sponsor or principal investigator of a clinical study is typically responsible for filing the document tracking.
How to fill out clinical study document tracking?
Clinical study document tracking can be filled out by ensuring all required documents are collected, organized, and stored according to regulatory guidelines.
What is the purpose of clinical study document tracking?
The purpose of clinical study document tracking is to ensure all necessary documents are maintained, accessible, and in compliance with regulatory requirements.
What information must be reported on clinical study document tracking?
Information such as study protocols, consent forms, adverse event reports, and other relevant documents must be reported on clinical study document tracking.
Fill out your clinical study document tracking online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Clinical Study Document Tracking is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















