Get the free Study Drug Administration
Show details
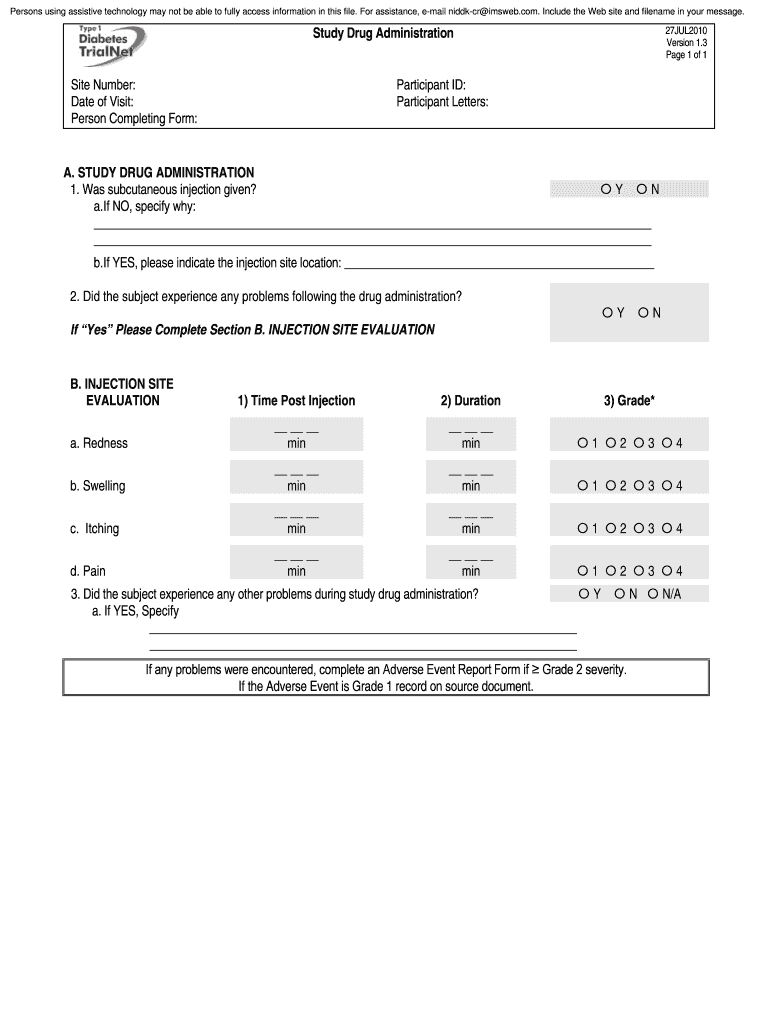
Persons using assistive technology may not be able to fully access information in this file. For assistance, email bidder imsweb.com. Include the Website and filename in your message. Study Drug Administration
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign study drug administration

Edit your study drug administration form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your study drug administration form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing study drug administration online
To use the services of a skilled PDF editor, follow these steps below:
1
Log in to your account. Start Free Trial and register a profile if you don't have one.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit study drug administration. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Save your file. Select it in the list of your records. Then, move the cursor to the right toolbar and choose one of the available exporting methods: save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud.
pdfFiller makes working with documents easier than you could ever imagine. Try it for yourself by creating an account!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out study drug administration

How to fill out study drug administration
01
Start by gathering all necessary information related to the study drug administration, such as the dosage instructions, timing, and any specific requirements or restrictions.
02
Prepare the study drug administration area by ensuring it is clean and organized. This may involve sanitizing surfaces, arranging necessary equipment and supplies, and setting up a comfortable and private space for the individual receiving the drug.
03
Verify the identity of the individual who needs the study drug administration to ensure it is the appropriate person and medication.
04
Follow proper safety protocols, such as wearing gloves and other personal protective equipment, as necessary.
05
Carefully measure or dispense the prescribed dosage of the study drug according to the provided instructions.
06
Administer the study drug to the individual using the approved route of administration. This may include oral, injectable, nasal, or other methods depending on the specific drug and its formulation.
07
Monitor the individual closely during and after drug administration for any adverse reactions or side effects.
08
Document the study drug administration process accurately and completely, including the dose administered, time, and any observations or complications.
09
Dispose of any leftover or unused study drugs properly, following the recommended disposal methods provided by the study protocol or healthcare facility.
10
Finally, ensure proper storage and handling of the study drug, following any specific instructions for temperature control, light exposure, or other relevant factors.
Who needs study drug administration?
01
Study drug administration is required for individuals participating in clinical trials or research studies that involve the use of experimental drugs or therapies.
02
These individuals may have specific medical conditions or be part of a control group receiving a placebo or standard treatment for comparison purposes.
03
The study drug administration is typically overseen by healthcare professionals or trained study coordinators to ensure safety and compliance with the study protocol.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I edit study drug administration from Google Drive?
It is possible to significantly enhance your document management and form preparation by combining pdfFiller with Google Docs. This will allow you to generate papers, amend them, and sign them straight from your Google Drive. Use the add-on to convert your study drug administration into a dynamic fillable form that can be managed and signed using any internet-connected device.
Can I edit study drug administration on an iOS device?
You certainly can. You can quickly edit, distribute, and sign study drug administration on your iOS device with the pdfFiller mobile app. Purchase it from the Apple Store and install it in seconds. The program is free, but in order to purchase a subscription or activate a free trial, you must first establish an account.
How do I complete study drug administration on an Android device?
Use the pdfFiller mobile app and complete your study drug administration and other documents on your Android device. The app provides you with all essential document management features, such as editing content, eSigning, annotating, sharing files, etc. You will have access to your documents at any time, as long as there is an internet connection.
What is study drug administration?
Study drug administration refers to the process of administering medication or treatment to participants in a research study or clinical trial.
Who is required to file study drug administration?
The individuals or organization running the research study or clinical trial are typically required to file study drug administration.
How to fill out study drug administration?
Study drug administration forms are typically filled out with details about the medication or treatment administered, dosage, frequency, and any observed side effects.
What is the purpose of study drug administration?
The purpose of study drug administration is to monitor the administration of medication or treatment in research studies or clinical trials to ensure participant safety and data accuracy.
What information must be reported on study drug administration?
Information such as participant ID, date and time of administration, medication name, dosage, and any side effects observed must be reported on study drug administration forms.
Fill out your study drug administration online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Study Drug Administration is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















