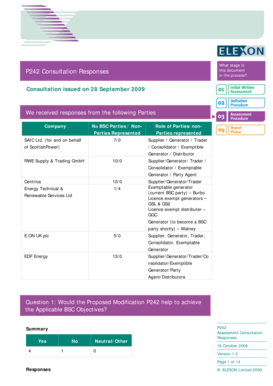
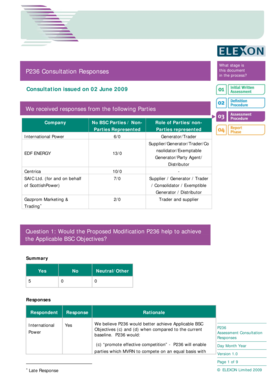
Get the free DEA publishes guidance on new training requirements for ...
Show details
RegistrationAttendee InformationFirst NameMiddle Initially Name MDDOYou will be asked to complete four educational outcomes surveys:Headdress City registering for this CME activity as a prescriber
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign dea publishes guidance on

Edit your dea publishes guidance on form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your dea publishes guidance on form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit dea publishes guidance on online
Use the instructions below to start using our professional PDF editor:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit dea publishes guidance on. Rearrange and rotate pages, insert new and alter existing texts, add new objects, and take advantage of other helpful tools. Click Done to apply changes and return to your Dashboard. Go to the Documents tab to access merging, splitting, locking, or unlocking functions.
4
Get your file. When you find your file in the docs list, click on its name and choose how you want to save it. To get the PDF, you can save it, send an email with it, or move it to the cloud.
With pdfFiller, it's always easy to work with documents. Try it out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out dea publishes guidance on

Point by point, here is how to fill out DEA publishes guidance on:
01
Read the guidance: Start by thoroughly reading the DEA publishes guidance document. This will help you understand the purpose, requirements, and any specific instructions provided.
02
Gather necessary information: Collect all the relevant information needed to fill out the guidance. This may include details about the controlled substances being discussed, any specific regulations or laws applicable, and any other supporting documentation required.
03
Understand the format: Familiarize yourself with the format in which the guidance needs to be filled out. Note any sections, fields, or specific instructions for each part of the form.
04
Follow guidelines: Make sure to adhere to any guidelines or instructions outlined in the guidance document. This may include specific formatting, language usage, or supporting documents to be included.
05
Complete each section: Proceed to complete each section of the guidance form accurately and thoroughly. Double-check the provided information for accuracy and completeness before moving on to the next section.
06
Review and revise: After completing the form, review it carefully to ensure all the information is accurate and all required fields have been filled out. Make any necessary revisions or corrections as needed.
07
Seek clarification if needed: If you come across any doubts or uncertainties while filling out the guidance, don't hesitate to seek clarification. Contact the appropriate DEA personnel or consult the guidance document itself for any contact details provided.
08
Submit the form: Once you are confident that the guidance form is accurately filled out, submit it as per the instructions provided. This may involve mailing it, submitting online through a portal, or any other specified method.
Who needs DEA publishes guidance on?
01
Individuals or organizations involved in the production, distribution, or handling of controlled substances.
02
Medical professionals, including doctors, pharmacists, and practitioners, who prescribe or dispense controlled substances.
03
Law enforcement agencies responsible for enforcing controlled substance laws and regulations.
04
Anyone involved in research or academic activities related to controlled substances.
05
Individuals or organizations interested in staying updated with the latest regulatory guidelines and practices concerning controlled substances.
Remember, it is essential to consult the specific DEA publishes guidance document to understand if it is applicable to your particular situation or industry.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I make changes in dea publishes guidance on?
With pdfFiller, you may not only alter the content but also rearrange the pages. Upload your dea publishes guidance on and modify it with a few clicks. The editor lets you add photos, sticky notes, text boxes, and more to PDFs.
Can I create an electronic signature for the dea publishes guidance on in Chrome?
Yes. By adding the solution to your Chrome browser, you can use pdfFiller to eSign documents and enjoy all of the features of the PDF editor in one place. Use the extension to create a legally-binding eSignature by drawing it, typing it, or uploading a picture of your handwritten signature. Whatever you choose, you will be able to eSign your dea publishes guidance on in seconds.
How do I complete dea publishes guidance on on an iOS device?
Get and install the pdfFiller application for iOS. Next, open the app and log in or create an account to get access to all of the solution’s editing features. To open your dea publishes guidance on, upload it from your device or cloud storage, or enter the document URL. After you complete all of the required fields within the document and eSign it (if that is needed), you can save it or share it with others.
What is dea publishes guidance on?
DEA publishes guidance on controlled substances.
Who is required to file dea publishes guidance on?
Anyone involved in handling controlled substances.
How to fill out dea publishes guidance on?
You can fill out DEA publishes guidance online or submit a paper form.
What is the purpose of dea publishes guidance on?
The purpose is to track the use and distribution of controlled substances.
What information must be reported on dea publishes guidance on?
Information such as type and quantity of controlled substances, recipient, and purpose.
Fill out your dea publishes guidance on online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Dea Publishes Guidance On is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.