
Get the free Clinical hold still in effect for Repros' oral drugBioPharma Dive
Show details
NCT02811159 Study ID: ZPU203EXTTitle: An OpenTable Extension Study of 12 mg Propelled (Telapristone Acetate) Administered Orally in the Treatment of Premenopausal Women with Confirmed Symptomatic
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign clinical hold still in
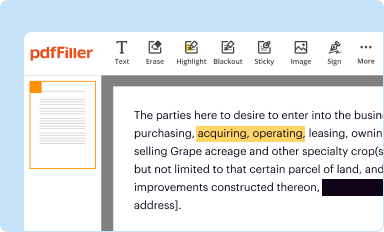
Edit your clinical hold still in form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.
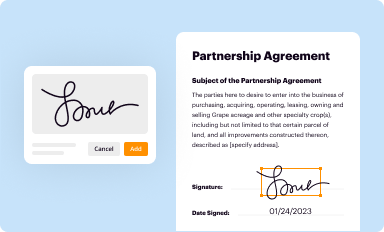
Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your clinical hold still in form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit clinical hold still in online
To use the professional PDF editor, follow these steps:
1
Create an account. Begin by choosing Start Free Trial and, if you are a new user, establish a profile.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit clinical hold still in. Rearrange and rotate pages, insert new and alter existing texts, add new objects, and take advantage of other helpful tools. Click Done to apply changes and return to your Dashboard. Go to the Documents tab to access merging, splitting, locking, or unlocking functions.
4
Save your file. Select it from your list of records. Then, move your cursor to the right toolbar and choose one of the exporting options. You can save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud, among other things.
pdfFiller makes working with documents easier than you could ever imagine. Register for an account and see for yourself!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out clinical hold still in
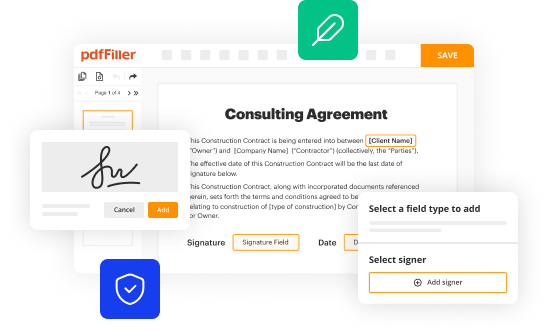
How to fill out clinical hold still in
01
Understand the reason for the clinical hold. It is important to know the specific requirements and regulations related to clinical hold in order to successfully fill out the necessary documentation.
02
Gather all the relevant information. Make sure you have all the necessary data, including study protocols, clinical data, adverse event reports, and any other supporting documents.
03
Review the clinical hold application form. Familiarize yourself with the format and specific sections that need to be completed.
04
Complete the application form accurately. Provide all the required information in a clear and concise manner. Use additional attachments or supporting documents if needed.
05
Ensure compliance with regulatory guidelines. Double-check that all the information provided meets the requirements set forth by the regulatory authorities.
06
Submit the completed application. Follow the provided instructions on how and where to submit the clinical hold application form.
07
Monitor the progress of the application. Stay in touch with the regulatory authorities to track the status of your clinical hold application.
08
Address any additional requests or inquiries. If the regulatory authorities require further information or clarification, respond promptly and provide the requested details.
09
Maintain open communication. Keep all relevant parties informed about the status and progress of the clinical hold fill-out process.
10
Seek professional assistance if needed. If you are unsure about any aspect of the clinical hold fill-out process, consider consulting with experts or regulatory consultants.
Who needs clinical hold still in?
01
Clinical hold is needed by pharmaceutical companies, researchers, or individuals conducting clinical trials.
02
It is required when there are concerns regarding the safety of the participants, the validity of the study data, or if any regulatory non-compliance is identified.
03
Regulatory authorities may impose a clinical hold to ensure the protection and well-being of the subjects enrolled in the clinical trial.
04
The clinical hold allows for further assessment and review of the trial before allowing it to proceed.
05
Overall, anyone involved in clinical trials should be aware of clinical hold processes and requirements.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I manage my clinical hold still in directly from Gmail?
It's easy to use pdfFiller's Gmail add-on to make and edit your clinical hold still in and any other documents you get right in your email. You can also eSign them. Take a look at the Google Workspace Marketplace and get pdfFiller for Gmail. Get rid of the time-consuming steps and easily manage your documents and eSignatures with the help of an app.
How can I send clinical hold still in to be eSigned by others?
Once you are ready to share your clinical hold still in, you can easily send it to others and get the eSigned document back just as quickly. Share your PDF by email, fax, text message, or USPS mail, or notarize it online. You can do all of this without ever leaving your account.
Can I edit clinical hold still in on an Android device?
With the pdfFiller Android app, you can edit, sign, and share clinical hold still in on your mobile device from any place. All you need is an internet connection to do this. Keep your documents in order from anywhere with the help of the app!
What is clinical hold still in?
Clinical hold is a status imposed by a regulatory agency on a drug development program or clinical trial, which indicates that the trial cannot proceed until the hold is removed.
Who is required to file clinical hold still in?
The sponsor or the individual or entity responsible for conducting the clinical trial is required to file the clinical hold.
How to fill out clinical hold still in?
The clinical hold must be filled out according to the specific guidelines provided by the regulatory agency overseeing the trial.
What is the purpose of clinical hold still in?
The purpose of clinical hold is to ensure the safety and rights of trial participants, as well as to protect the integrity of the data generated from the trial.
What information must be reported on clinical hold still in?
The clinical hold must report the reasons for imposing the hold, the actions taken to address the issues, and the plans for resolving the hold.
Fill out your clinical hold still in online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Clinical Hold Still In is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















