Get the free FORM FDA 3511-3. Aseptic Processing and Packaging Report
Show details
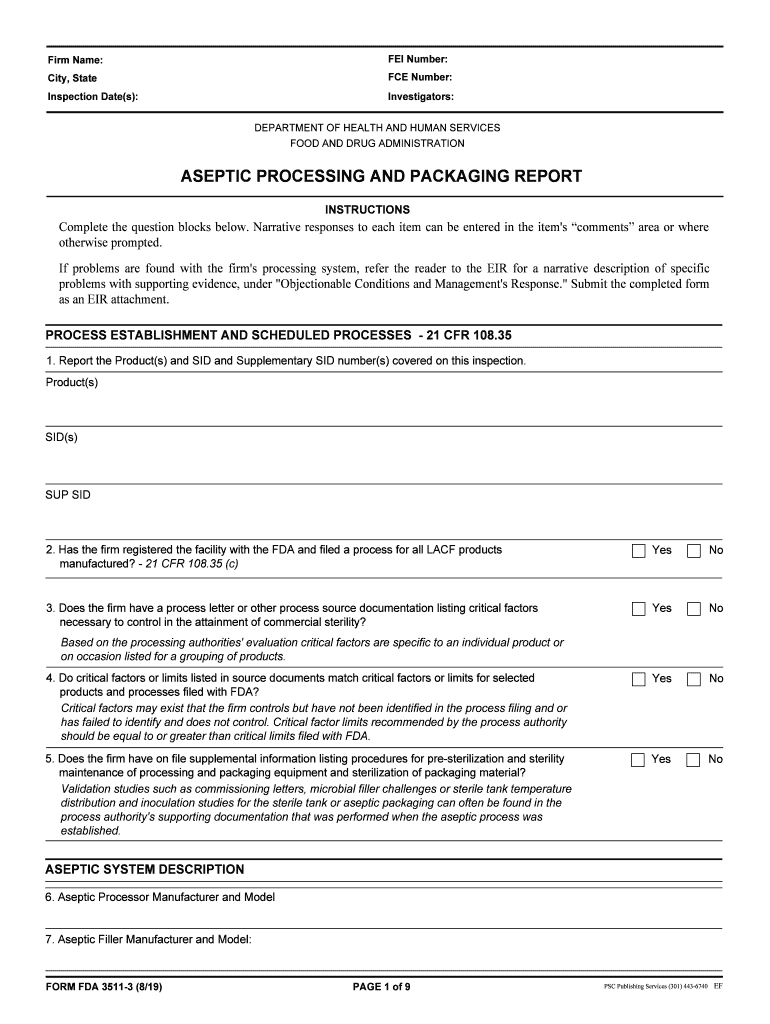
Firm Name:FEI Number:City, State FCE Number:Inspection Date(s):Investigators:
DEPARTMENT OF HEALTH AND HUMAN SERVICES
FOOD AND DRUG ADMINISTRATIONASEPTIC PROCESSING AND PACKAGING REPORT
INSTRUCTIONSComplete
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign form fda 3511-3 aseptic

Edit your form fda 3511-3 aseptic form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your form fda 3511-3 aseptic form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit form fda 3511-3 aseptic online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Log in to account. Start Free Trial and sign up a profile if you don't have one.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit form fda 3511-3 aseptic. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
It's easier to work with documents with pdfFiller than you can have ever thought. You may try it out for yourself by signing up for an account.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out form fda 3511-3 aseptic

How to fill out form fda 3511-3 aseptic
01
Start by downloading the FDA 3511-3 Aseptic form from the official FDA website.
02
Read the instructions carefully to understand the requirements for filling out the form.
03
Begin by providing your personal information in the designated sections of the form, such as your name, address, and contact information.
04
Specify the purpose of the form and provide any necessary details or explanations.
05
Follow the form's structure and fill in each section accurately and thoroughly.
06
If there are any additional documents or attachments required, ensure you include them properly according to the instructions.
07
Once you have completed filling out the form, review it carefully to ensure all information is accurate and all required fields are completed.
08
If necessary, seek professional guidance or assistance to verify the accuracy and completeness of the form.
09
Sign and date the form as required, indicating your agreement and understanding of the provided information.
10
Make copies of the completed form and any supporting documents for your records.
11
Submit the filled-out form and any necessary supporting documents as instructed by the FDA.
Who needs form fda 3511-3 aseptic?
01
The FDA 3511-3 Aseptic form is required by individuals or organizations who are involved in aseptic processing of food, drugs, cosmetics, or medical devices.
02
This form is specifically designed for those who need to comply with the FDA's regulations and guidelines for aseptic processing.
03
It may be required by manufacturers, packagers, distributors, importers, or any entity involved in the production or distribution of aseptically processed products.
04
It is crucial to consult the FDA guidelines or seek professional advice to determine if you need to fill out the FDA 3511-3 Aseptic form for your specific situation.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
Where do I find form fda 3511-3 aseptic?
It’s easy with pdfFiller, a comprehensive online solution for professional document management. Access our extensive library of online forms (over 25M fillable forms are available) and locate the form fda 3511-3 aseptic in a matter of seconds. Open it right away and start customizing it using advanced editing features.
How do I edit form fda 3511-3 aseptic in Chrome?
Download and install the pdfFiller Google Chrome Extension to your browser to edit, fill out, and eSign your form fda 3511-3 aseptic, which you can open in the editor with a single click from a Google search page. Fillable documents may be executed from any internet-connected device without leaving Chrome.
Can I sign the form fda 3511-3 aseptic electronically in Chrome?
You certainly can. You get not just a feature-rich PDF editor and fillable form builder with pdfFiller, but also a robust e-signature solution that you can add right to your Chrome browser. You may use our addon to produce a legally enforceable eSignature by typing, sketching, or photographing your signature with your webcam. Choose your preferred method and eSign your form fda 3511-3 aseptic in minutes.
What is form FDA 3511-3 aseptic?
FDA Form 3511-3 aseptic is a form used to report information related to aseptic processing activities in the manufacturing of drugs or biological products.
Who is required to file form FDA 3511-3 aseptic?
Manufacturers of drugs or biological products who engage in aseptic processing activities are required to file form FDA 3511-3 aseptic.
How to fill out form FDA 3511-3 aseptic?
Form FDA 3511-3 aseptic can be filled out electronically or manually following the instructions provided by the FDA.
What is the purpose of form FDA 3511-3 aseptic?
The purpose of form FDA 3511-3 aseptic is to ensure the proper reporting of information related to aseptic processing activities in order to maintain the safety and efficacy of drugs or biological products.
What information must be reported on form FDA 3511-3 aseptic?
Information such as aseptic processing methods, equipment used, validation practices, and environmental monitoring data must be reported on form FDA 3511-3 aseptic.
Fill out your form fda 3511-3 aseptic online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Form Fda 3511-3 Aseptic is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.