Get the free CLINICAL TRIAL OF AN IMPLANTABLE CARDIAC DEFIBRILLATOR
Show details
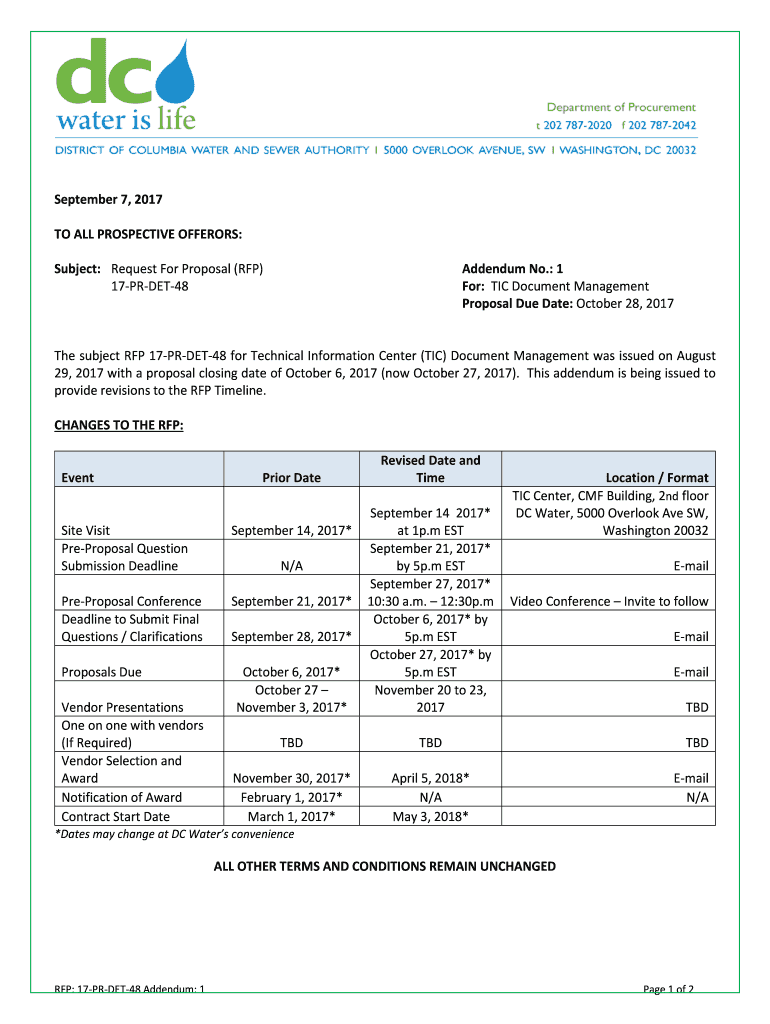
September 7, 2017,
TO ALL PROSPECTIVE OFFER ORS:
Subject: Request For Proposal (RFP)
17PRDET48Addendum No.: 1
For: TIC Document Management
Proposal Due Date: October 28, 2017The subject RFP 17PRDET48
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign clinical trial of an

Edit your clinical trial of an form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your clinical trial of an form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing clinical trial of an online
Follow the guidelines below to use a professional PDF editor:
1
Create an account. Begin by choosing Start Free Trial and, if you are a new user, establish a profile.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit clinical trial of an. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Save your file. Select it from your list of records. Then, move your cursor to the right toolbar and choose one of the exporting options. You can save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud, among other things.
pdfFiller makes dealing with documents a breeze. Create an account to find out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out clinical trial of an

How to fill out clinical trial of an
01
Step 1: Start by carefully reading the instructions and requirements provided with the clinical trial form. Make sure you understand what information is needed and how to fill it out correctly.
02
Step 2: Begin by entering your basic personal details such as your name, contact information, age, and gender.
03
Step 3: Provide your medical history, including any pre-existing conditions, previous treatments, and surgeries.
04
Step 4: Next, fill out the consent section, indicating your willingness to participate in the clinical trial and any associated risks or benefits you have been informed about.
05
Step 5: Complete the questionnaire or survey portion of the form, answering all questions honestly and accurately.
06
Step 6: If required, provide additional documents or medical reports as requested by the clinical trial organizers.
07
Step 7: Review your filled-out form for any errors or missing information before submitting it.
08
Step 8: Sign and date the form as required, indicating your agreement to participate in the clinical trial.
09
Step 9: Submit your completed form to the designated person or organization responsible for collecting the clinical trial applications.
10
Step 10: Await further communication from the clinical trial organizers regarding your eligibility and participation in the trial.
Who needs clinical trial of an?
01
Clinical trials are typically needed by individuals who are interested in advancing medical research and finding potential treatments or cures for specific conditions or diseases.
02
Patients who have exhausted standard treatment options or have conditions for which no cure or effective treatment currently exists may also seek clinical trials as a potential avenue for accessing experimental therapies.
03
Additionally, healthy individuals may participate in clinical trials to contribute to scientific knowledge and help researchers understand the safety and efficacy of new treatments.
04
The specific eligibility criteria for each clinical trial may vary, so it is important to review the trial's requirements to determine if you meet the specified criteria and if the trial is suitable for your individual circumstances.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I get clinical trial of an?
With pdfFiller, an all-in-one online tool for professional document management, it's easy to fill out documents. Over 25 million fillable forms are available on our website, and you can find the clinical trial of an in a matter of seconds. Open it right away and start making it your own with help from advanced editing tools.
How do I edit clinical trial of an straight from my smartphone?
Using pdfFiller's mobile-native applications for iOS and Android is the simplest method to edit documents on a mobile device. You may get them from the Apple App Store and Google Play, respectively. More information on the apps may be found here. Install the program and log in to begin editing clinical trial of an.
How do I complete clinical trial of an on an iOS device?
Install the pdfFiller iOS app. Log in or create an account to access the solution's editing features. Open your clinical trial of an by uploading it from your device or online storage. After filling in all relevant fields and eSigning if required, you may save or distribute the document.
What is clinical trial of an?
Clinical trial of an is a process of testing a new treatment or medical procedure on human subjects to determine its safety and effectiveness.
Who is required to file clinical trial of an?
Researchers, pharmaceutical companies, and other organizations conducting clinical trials are required to file clinical trial of an.
How to fill out clinical trial of an?
Clinical trial of an can be filled out online through the designated government website by providing all relevant information about the trial.
What is the purpose of clinical trial of an?
The purpose of clinical trial of an is to ensure transparency and accountability in the testing of new treatments and procedures on human subjects.
What information must be reported on clinical trial of an?
Information such as study objectives, methodology, anticipated risks, and informed consent procedures must be reported on clinical trial of an.
Fill out your clinical trial of an online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Clinical Trial Of An is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.




















