Get the Entropy, Spontaneity, and Free Energy - web mnstate
Show details
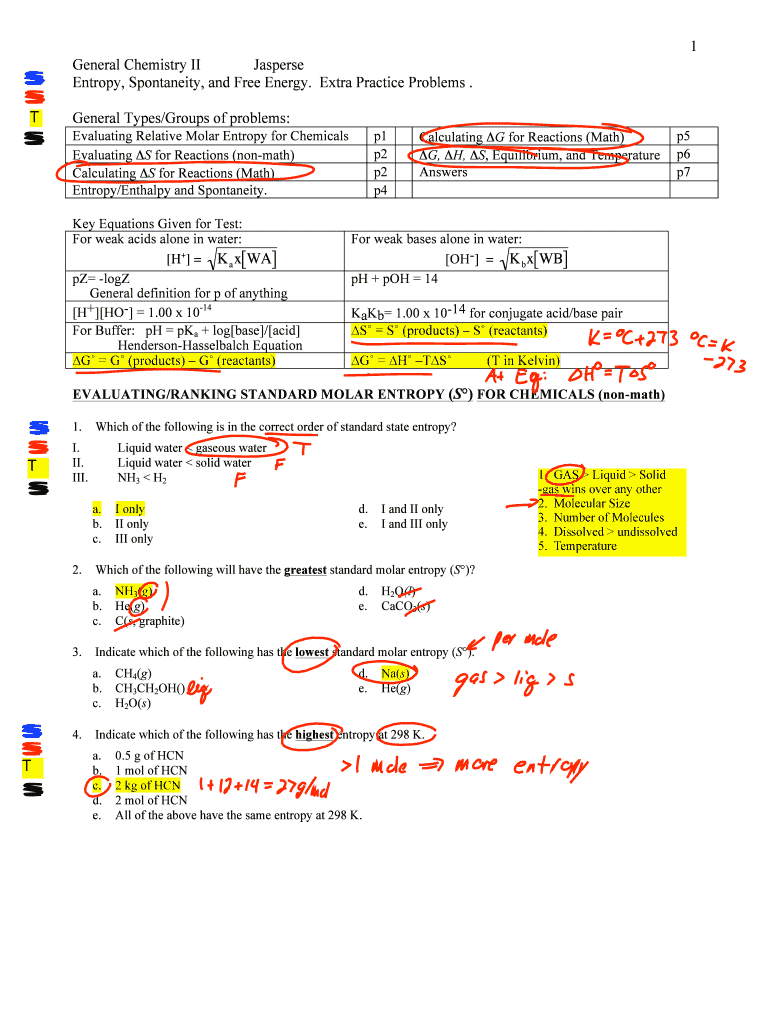
1 General Chemistry II Jasper Entropy, Spontaneity, and Free Energy. Extra Practice Problems. General Types/Groups of problems: Evaluating Relative Molar Entropy for Chemicals Evaluating S for Reactions
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign entropy spontaneity and energy

Edit your entropy spontaneity and energy form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your entropy spontaneity and energy form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing entropy spontaneity and energy online
To use our professional PDF editor, follow these steps:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit entropy spontaneity and energy. Replace text, adding objects, rearranging pages, and more. Then select the Documents tab to combine, divide, lock or unlock the file.
4
Get your file. When you find your file in the docs list, click on its name and choose how you want to save it. To get the PDF, you can save it, send an email with it, or move it to the cloud.
Dealing with documents is always simple with pdfFiller.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out entropy spontaneity and energy

How to fill out entropy spontaneity and energy
01
To fill out entropy, spontaneity, and energy, follow these steps:
02
Start by understanding the concept of entropy. Entropy measures the disorder or randomness in a system. It is represented by the symbol 'S'.
03
Next, learn about spontaneity. Spontaneous processes occur naturally without any external influence. They tend to increase the overall entropy of the system.
04
Now, focus on energy. Energy is the ability to do work or cause change. It can exist in different forms such as thermal, kinetic, potential, etc.
05
Understand the relationship between entropy, spontaneity, and energy. In general, processes that increase entropy are often spontaneous and associated with a decrease in energy.
06
Apply the laws of thermodynamics to analyze these concepts. The first law of thermodynamics states that energy is conserved in a system, while the second law of thermodynamics relates to entropy and spontaneity.
07
Use mathematical equations and formulas to calculate entropy, spontaneity, and energy in specific systems. These calculations typically involve factors such as temperature, pressure, and the number of particles.
08
Finally, interpret and analyze the results. Understanding entropy, spontaneity, and energy enables scientists and engineers to predict and control various physical, chemical, and biological processes.
Who needs entropy spontaneity and energy?
01
Entropy, spontaneity, and energy are fundamental concepts in various fields of science and engineering. They are needed by:
02
- Chemists, to understand and predict chemical reactions and equilibrium.
03
- Physicists, to analyze thermodynamic systems and study the behavior of matter and energy.
04
- Biologists, to comprehend biological processes and the transfer of energy within living organisms.
05
- Engineers, to design and optimize energy-efficient systems and processes.
06
- Environmental scientists, to assess the impact of energy transformations on ecosystems and the environment.
07
- Researchers in materials science, to investigate the properties and behavior of materials at different energy and entropy levels.
08
- Students studying science and engineering disciplines, as these concepts form the foundation of their education and research.
09
In summary, entropy, spontaneity, and energy are relevant in a wide range of scientific and engineering fields, and anyone involved in understanding, analyzing, and manipulating these processes requires a knowledge of these concepts.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I edit entropy spontaneity and energy online?
The editing procedure is simple with pdfFiller. Open your entropy spontaneity and energy in the editor, which is quite user-friendly. You may use it to blackout, redact, write, and erase text, add photos, draw arrows and lines, set sticky notes and text boxes, and much more.
How do I fill out the entropy spontaneity and energy form on my smartphone?
Use the pdfFiller mobile app to complete and sign entropy spontaneity and energy on your mobile device. Visit our web page (https://edit-pdf-ios-android.pdffiller.com/) to learn more about our mobile applications, the capabilities you’ll have access to, and the steps to take to get up and running.
How do I complete entropy spontaneity and energy on an Android device?
On an Android device, use the pdfFiller mobile app to finish your entropy spontaneity and energy. The program allows you to execute all necessary document management operations, such as adding, editing, and removing text, signing, annotating, and more. You only need a smartphone and an internet connection.
What is entropy spontaneity and energy?
Entropy spontaneity and energy refer to the thermodynamic properties that govern the spontaneity and direction of chemical reactions.
Who is required to file entropy spontaneity and energy?
Scientists, researchers, and professionals working in the field of thermodynamics are typically required to file entropy spontaneity and energy.
How to fill out entropy spontaneity and energy?
Entropy spontaneity and energy can be filled out by analyzing the data obtained from experiments and calculations based on thermodynamic principles.
What is the purpose of entropy spontaneity and energy?
The purpose of entropy spontaneity and energy is to understand the energy changes and the likelihood of a chemical reaction occurring spontaneously.
What information must be reported on entropy spontaneity and energy?
Information such as initial and final energy states, temperature, pressure, and the nature of the reactants and products must be reported on entropy spontaneity and energy.
Fill out your entropy spontaneity and energy online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Entropy Spontaneity And Energy is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.




















