Get the free Vaccine Cold-Chain Monitoring - dchealth dc
Show details
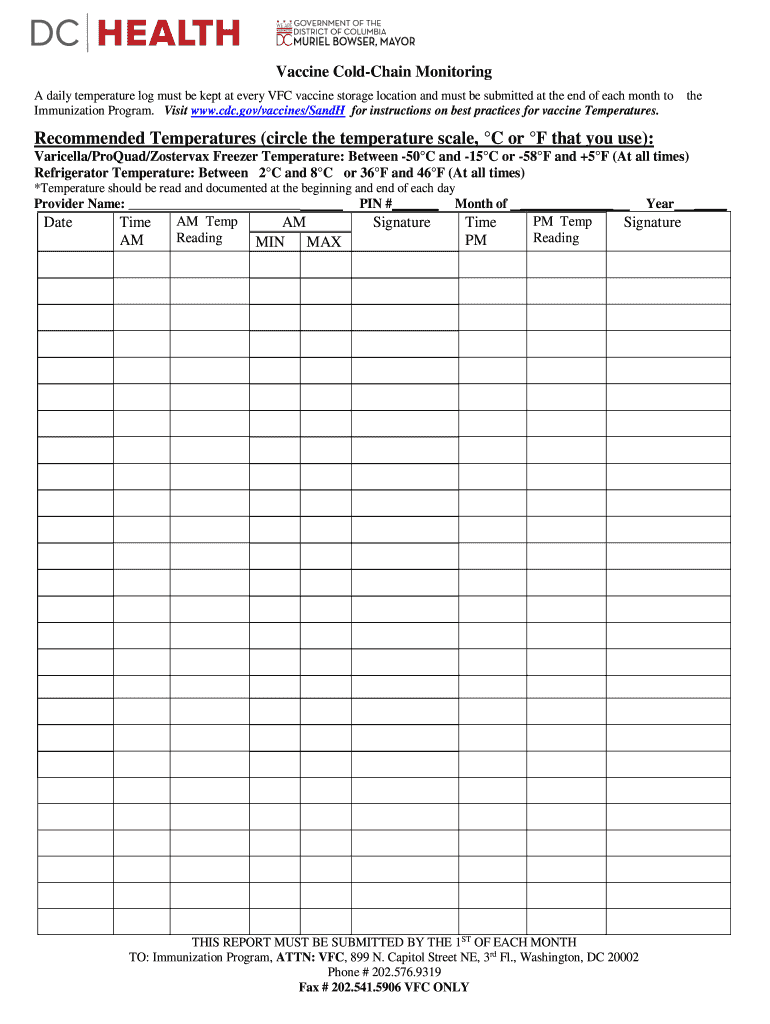
Vaccine Cochin Monitoring
A daily temperature log must be kept at every AFC vaccine storage location and must be submitted at the end of each month to
Immunization Program. Visit www.cdc.gov/vaccines/SandH
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign vaccine cold-chain monitoring

Edit your vaccine cold-chain monitoring form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your vaccine cold-chain monitoring form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing vaccine cold-chain monitoring online
To use the professional PDF editor, follow these steps below:
1
Create an account. Begin by choosing Start Free Trial and, if you are a new user, establish a profile.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit vaccine cold-chain monitoring. Rearrange and rotate pages, add new and changed texts, add new objects, and use other useful tools. When you're done, click Done. You can use the Documents tab to merge, split, lock, or unlock your files.
4
Save your file. Select it in the list of your records. Then, move the cursor to the right toolbar and choose one of the available exporting methods: save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud.
pdfFiller makes dealing with documents a breeze. Create an account to find out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out vaccine cold-chain monitoring

How to fill out vaccine cold-chain monitoring
01
To fill out vaccine cold-chain monitoring, follow these steps:
02
Ensure that you have the required monitoring forms and documentation.
03
Begin by recording the starting temperature of the vaccine storage unit.
04
Check the temperature of the storage unit at regular intervals, such as every hour or as per the guidelines provided.
05
Record the temperature reading accurately on the monitoring form.
06
Take necessary actions if the temperature goes outside the recommended range, such as adjusting the thermostat or transferring vaccines to another storage unit.
07
Continue monitoring and recording temperatures throughout the storage period.
08
At the end of the storage period, calculate the average temperature and ensure it falls within the acceptable range.
09
Complete any additional required sections on the monitoring form, such as vaccine identification, date, and time.
10
Submit the filled-out monitoring form as per the designated process.
11
Keep a copy of the completed form for your records.
Who needs vaccine cold-chain monitoring?
01
Vaccine cold-chain monitoring is crucial for various stakeholders involved in the vaccine supply chain:
02
- Vaccine manufacturers: They need to ensure the quality and efficacy of their vaccines during storage and transportation.
03
- Distributors and logistics providers: They are responsible for maintaining appropriate temperature conditions to prevent vaccine spoilage.
04
- Healthcare facilities and providers: They need to store and handle vaccines correctly to ensure their effectiveness and safety for patients.
05
- Regulatory bodies: They require monitoring data to enforce compliance with cold-chain guidelines and regulations.
06
- Public health officials: They rely on accurate monitoring to assess vaccine viability, plan immunization programs, and prevent vaccine wastage.
07
- Quality assurance personnel: They use monitoring data to identify any deviations from the required temperature range and take corrective actions.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I send vaccine cold-chain monitoring to be eSigned by others?
To distribute your vaccine cold-chain monitoring, simply send it to others and receive the eSigned document back instantly. Post or email a PDF that you've notarized online. Doing so requires never leaving your account.
Can I create an eSignature for the vaccine cold-chain monitoring in Gmail?
Create your eSignature using pdfFiller and then eSign your vaccine cold-chain monitoring immediately from your email with pdfFiller's Gmail add-on. To keep your signatures and signed papers, you must create an account.
How do I edit vaccine cold-chain monitoring straight from my smartphone?
You may do so effortlessly with pdfFiller's iOS and Android apps, which are available in the Apple Store and Google Play Store, respectively. You may also obtain the program from our website: https://edit-pdf-ios-android.pdffiller.com/. Open the application, sign in, and begin editing vaccine cold-chain monitoring right away.
What is vaccine cold-chain monitoring?
Vaccine cold-chain monitoring is the process of tracking and ensuring the proper storage and handling of vaccines to maintain their effectiveness.
Who is required to file vaccine cold-chain monitoring?
Healthcare facilities, vaccine manufacturers, and distributors are required to file vaccine cold-chain monitoring.
How to fill out vaccine cold-chain monitoring?
Vaccine cold-chain monitoring can be filled out by documenting temperature logs, storage conditions, and handling procedures of vaccines.
What is the purpose of vaccine cold-chain monitoring?
The purpose of vaccine cold-chain monitoring is to prevent vaccines from being exposed to temperatures that could reduce their potency and effectiveness.
What information must be reported on vaccine cold-chain monitoring?
Information such as date and time of temperature recordings, storage equipment used, and any temperature excursions must be reported on vaccine cold-chain monitoring.
Fill out your vaccine cold-chain monitoring online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Vaccine Cold-Chain Monitoring is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.




















